Lipid nanoparticles in drug development
Lipid nanoparticles (LNPs) have emerged as promising carriers for nucleic-acid-based therapeutics and vaccines. Their design and application depend heavily on structural and morphological properties, which directly influence their function, safety, and efficacy. Comprehensive characterization is crucial for designing effective lipid nanoparticle formulations. In this blog, we explore the importance of this characterization and the role of cryo-transmission electron microscopy (cryo-TEM) in the assessment of LNPs.
Advantages of cryo-TEM in lipid nanoparticle characterization
Traditional analytical techniques such as dynamic light scattering (DLS) and nanoparticle tracking analysis provide bulk measurement of size and distribution for LNPs, but lack the resolution needed to visualize individual nanoparticle structures. Cryo-TEM, on the other hand, allows researchers to directly observe LNPs in their native, hydrated state without the need for staining or fixation. This high-resolution imaging capability enables:
- Accurate size and shape analysis: cryo-TEM captures detailed images of individual LNPs, revealing the size distribution, polydispersity, and shape heterogeneity that impact drug delivery performance.
- Encapsulation efficiency assessment: cryo-TEM can reveal RNA loading and distribution within LNPs, providing critical information on formulation efficiency.
- Improvement of batch-to-batch consistency: manufacturing process variability can be assessed with cryo-TEM, helping to ensure reproducibility and product quality.
- Nano-structural insights: understanding of lipid phase behavior and the internal structure of LNPs aids in the optimization of formulation conditions for stability and efficacy.
- Process optimization: insights from cryo-TEM enable refinements in microfluidic mixing, extrusion, and other LNP manufacturing techniques.
Novel particle characterization with cryo-TEM is helping to advance our understanding of LNP structure, which is critical for optimizing formulations, improving RNA delivery efficiency, and developing next-generation nanomedicines.
Understanding structure-activity relationships of LNPs
Optimization of LNPs has focused on a variety of potential components that could improve transfection efficiency, including ionizable lipids, helper lipids, and PEG-lipids. However, the role of specific lipid structures in LNP-endosome interactions, and in RNA delivery to the cytoplasm, is still not fully understood.
To address this, scientists at the University of Bristol and Leiden University have taken a rational design approach to engineer LNPs with defined lipid superstructures.1 Using advanced imaging techniques such as cryo-electron tomography (cryo-ET), they revealed that LNPs can adopt distinct internal structures, including lamellar, liquid crystalline inverse hexagonal, and mixed lipid-RNA phases, each of which play a crucial role in transfection efficiency (Figure 1). This approach also enabled the differentiation between empty LNPs and those encapsulating siRNA, providing critical insights for the design optimization of LNPs and the improvement of RNA therapeutics (Figure 2).
This research highlights the power that direct cryo-TEM visualization can have in advancing our understanding of LNP structure-function relationships.
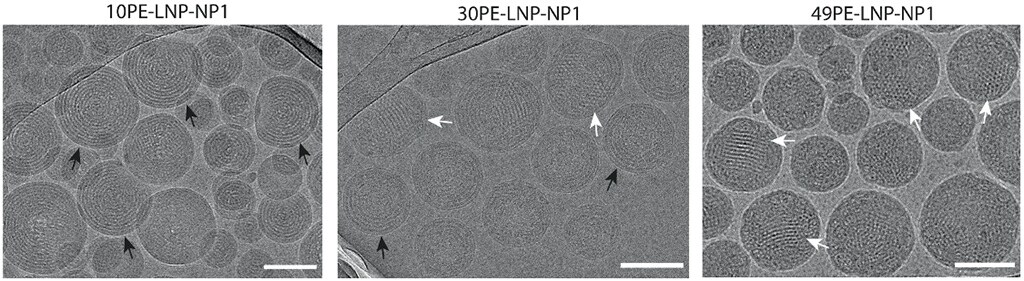
Figure 1. Representative cryo-TEM images of various lipid nanoparticle formulations. Black arrows highlight lamellar structures, while white arrows indicate non-lamellar structures. Scale bar = 100 nm. Figure reproduced from Pattipeiluhu et al. under CC BY 4.0.
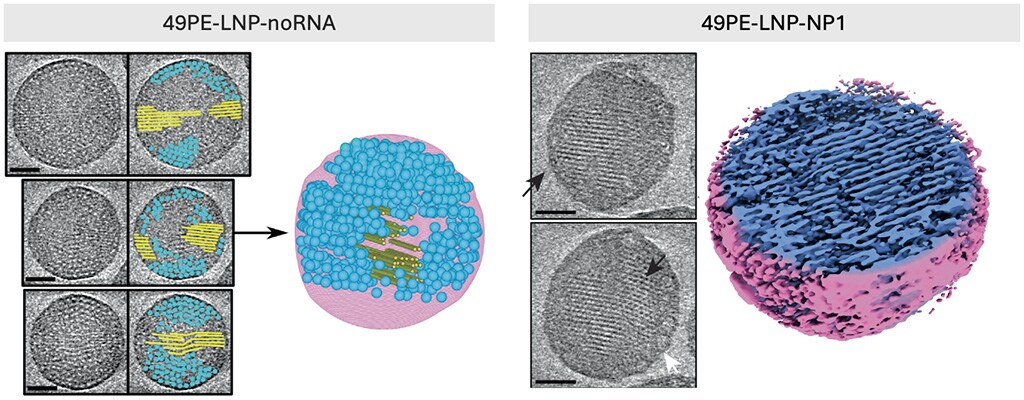
Figure 2. Ultrastructural cryo-ET analysis of empty (49PE-LNP-noRNA) and filled (49PE-LNP-NP1) LNPs. Scale bar = 50 nm. Figure reproduced from Pattipeiluhu et al. under CC BY 4.0.
Tackling the challenge of endosomal escape
LNP mRNA complexes are transforming targeted therapeutic delivery, yet challenges like inefficient endosomal escape, high toxicity, and prolonged tissue persistence have limited their applications. Recently, several studies have proposed solutions to improve endosomal escape.
A team of scientists at Shenzhen Neocurna Biotechnology Corporation introduced a novel cationic polymeric micelle (cPM) for co-delivery with LNPs, which was shown to improve endosomal escape and increase in vivo mRNA expression without altering the properties of the LNPs.2 Cryo-TEM played a vital role in confirming the structural integrity of the cPMs and LNPs, providing detailed insights into nanoparticle morphology, size distribution, and surface characteristics (Figure 3). The images revealed that cPMs had a nearly spherical shape with a rough surface, and their particle sizes were smaller than those measured by DLS. This discrepancy is likely due to cPM aggregation in solution, while cryo-TEM delivers a more accurate particle size by imaging the sample at the single-particle level in a vitrified state. This precision was essential for validating the quality and consistency of the formulations.
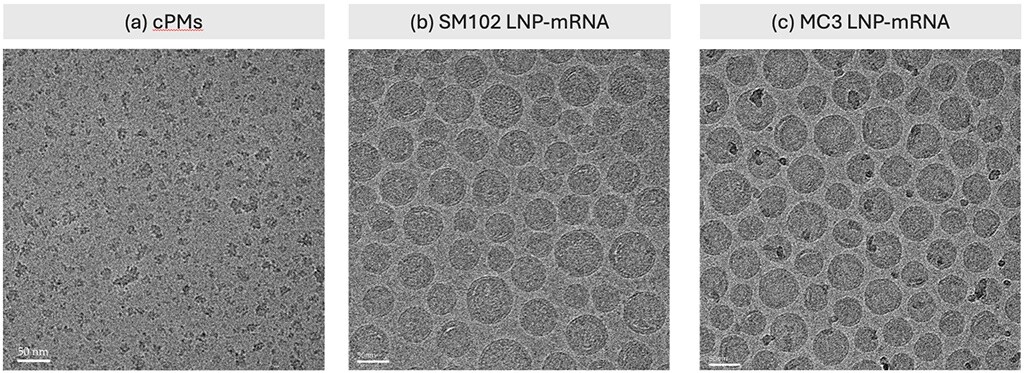
Figure 3. Representative cryo-TEM images of cPM micelles, which exhibit a rough surface, along with two LNP formulations displaying a well-defined spherical morphology. Scale bar = 50 nm. Figure reproduced from Deng et al. under CC BY 4.0.
Advancing the manufacturing of LNPs with accurate particle characterization
Developing scalable and reproducible methods for lipid nanoparticle production poses a significant challenge in nanomedicine. Visualization of LNPs at single particle level with cryo-TEM has proven to be pivotal, enabling researchers to meticulously compare various manufacturing methods and technologies with high detail and precision, thereby facilitating the optimization of LNP production processes.
A recent study by scientists at Leiden University and Johannes Gutenberg University introduced a cost-effective microfluidic platform, called CD, for the synthesis of mRNA LNPs, which allows for precise adjustments without compromising quality.3 Cryo-TEM analysis of these mRNA LNPs revealed that microfluidic-produced LNPs were smaller, more uniform, and featured ordered electron-dense cores. Buffer and ionic strength affected bleb formation and transfection efficacy, while PEG-lipid concentration influenced particle size.
New therapeutic approaches in cancer treatment and protein-replacement therapy
The rapid success of mRNA vaccines has positioned LNPs as a powerful delivery vehicle for nucleic acids. LNPs are also being explored for targeted treatments of cancer and metabolic diseases, which would require formulations that are optimized for repeated dosing with minimal immunogenicity.
Researchers at the Icahn School of Medicine at Mount Sinai have developed a novel LNP-RNA antigen presentation platform that repurposes SARS-CoV-2 vaccine-induced T-cell immunity to target cancer.4 By designing a series of amino-acid and amino-alcohol derived ionizable lipids (AA lipids), they were able to produce LNP formulations that efficiently deliver directly into tumors. Cryo-TEM played a critical role in visualizing the structural integrity, morphology, and encapsulation efficiency of these engineered LNPs.
In another study, scientists at the Johannes Gutenberg University and the Max Planck Institute for Polymer Research examined how LNP composition impacts mRNA encapsulation, stability, immune response, and biodistribution.5 Cryo-TEM showed that altering of individual LNP components resulted in distinct crystallographic ultrastructures, including bilamellar formations, “blebs,” and multilamellar particles, which directly impact the function and stability of the LNPs (Figure 4).

Figure 4. Cryo-TEM images of the various LNP formulations, with arrows highlighting specific structural features and irregularities, including “blebs” (green arrows) and internal defects (blue arrows).
Conclusions
LNPs are complex systems, and various nucleic acid cargo types and lipid modifications can create LNPs with distinct properties. Cryo-TEM enables the analysis of LNPs at the particle level, making it crucial for their development and characterization.
With increasing regulatory scrutiny on nanoparticle-based drug delivery, cryo-TEM has become an essential tool for the accurate assessment of critical quality attributes. Advancements in automated image processing, machine learning, and correlative microscopy, encompassed by comprehensive software solutions such as Thermo Scientific Amira Software, further enhance the role that cryo-TEM can play in the optimization of LNPs, helping to ensure the creation of safer and more effective therapeutics.
References
- Pattipeiluhu, R, et al.Liquid crystalline inverted lipid phases encapsulating siRNA enhance lipid nanoparticle mediated transfection. Nat Commun 15, 1303 (2024). doi: 1038/s41467-024-45666-5
- Deng, S, et al. Development of a Cationic Polymeric Micellar Structure with Endosomal Escape Capability Enables Enhanced Intramuscular Transfection of mRNA-LNPs. Vaccines13:25 (2025). doi: 3390/vaccines13010025
- Johnson, NV, et al. On the Influence of Fabrication Methods and Materials for mRNA-LNP Production: From Size and Morphology to Internal Structure and mRNA Delivery Performance In Vitro and In Vivo. Adv Healthc Mater 13:26 (2024). doi: 1002/adhm.202401252
- Xue, Y, et al. LNP-RNA-mediated antigen presentation leverages SARS-CoV-2-specific immunity for cancer treatment. Nat Commun 16:2198 (2025). doi: 1038/s41467-025-57149-2
- Gambaro, R, et al. Optimizing mRNA-Loaded Lipid Nanoparticles as a Potential Tool for Protein-Replacement Therapy.Pharmaceutics 16:771 (2024). doi: 3390/pharmaceutics16060771




Wow: amazing technology! It shows processes at nano level in real time. Medical treatments will herewith improve and increase their effectiveness.
We would like to be involved.