Cryo-electron microscopy (cryo-EM) is helping unravel the mysteries behind a wide range of diseases from Zika to neurodegenerative diseases. We recently sat down with Dr. Vinothkumar Kutti Ragunath, Assistant Professor at the NCBS Bangalore Life Science Cluster in India to learn how cryo-EM is helping his organization get a larger picture of how membrane proteins function and how they contribute to disease. Watch the interview below or continue reading for excerpts from our conversation.
This Q&A has been edited for clarity and length.
What inspired you to work on the biology of the brain?
My interest in the brain as an organ started because some of my cousins had epilepsy, and I was curious from a young age to understand what was happening. The recent advances in cryo-EM will possibly help us to understand how this network of neurons in the brain is functioning and how the signals are transmitted. These advances may also help us find some clues for many other diseases.
What are your primary research objectives?
My primary research objective here in Bangalore is focusing on membrane protein structure and dynamics, in particular on enzymes and ion channels in Eukaryotic cells. Also, because I am running a national facility, I cater to the needs of other researchers on campus who work on various things including ribosomes and proteins associated with ribosomes involved in translation which indirectly cause diseases.
How can understanding the membrane protein structure help patients in the long term?
More than 40-50 percent of drugs currently on the pharma drug market are targeted at membrane proteins simply because these proteins are involved in a lot of signalling that indirectly results in diseases like cancer, neurodegenerative diseases, etc. Knowledge of the structure of membrane proteins is going to help us understand how these proteins function, which will, in turn, help us design drugs that help patients with various diseases. It’s as if you are in a room that’s very dark and there are multiple doors, and you have bunch of keys but don’t know which key to use to open which door. It’s similar with these structures. If you know exactly where things are, you’re able to focus on developing a drug targeting a particular portion of the structure or protein to help the patient. This is why membrane protein structures are very critical for drug discovery.

Dr. Vinothkumar working in his lab.
Which cryo-EM application is of particular interest to your research?
With cryo-EM, we can do a lot of things including examining proteins as single molecules, the inside of cells, and small crystals. Currently, the most commonly used technique is single-particle EM, which is very popular among all structural biologists. But in the next 5 to 10 years, I see cryo-tomography, where we look at sections of a cell or sometimes even small bacterial cells, becoming more common. So, too, will micro electron diffraction (MicroED), in which small crystals that don’t diffract very well in an x-ray beam can be used to obtain structures. I think all three techniques are going to be very useful not only to structural biologists, but also to cell biologists, neurobiologists and anybody who wants to image a section of the cell.
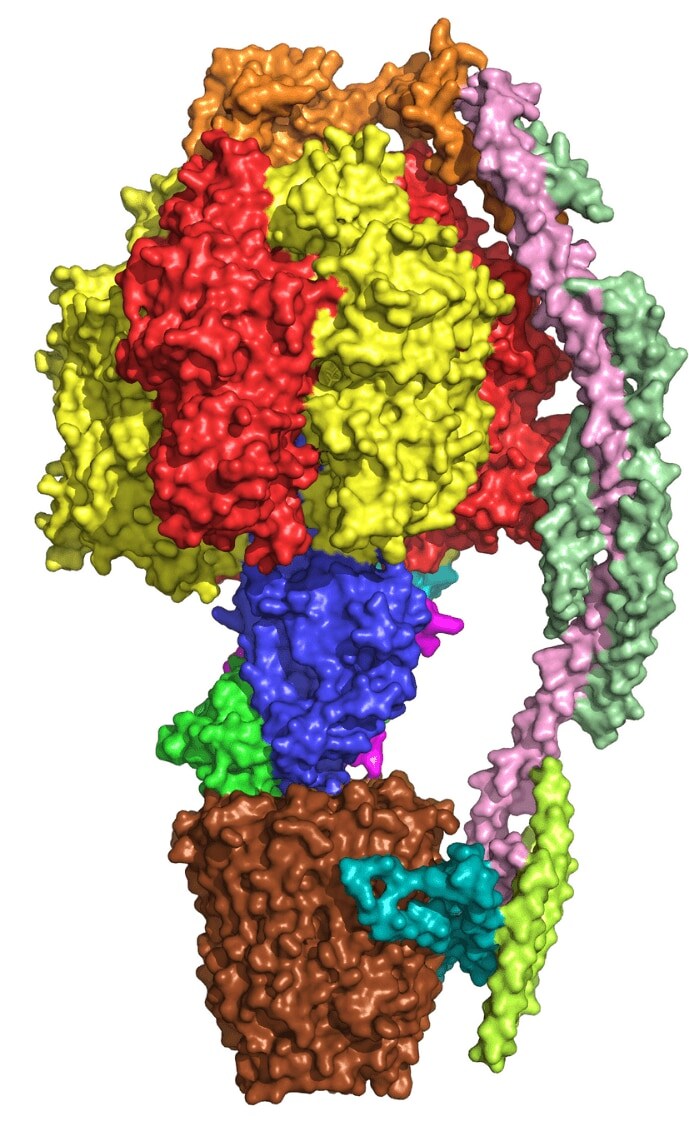
ATP synthase: The subunits of this yeast ATP synthase enzyme model are color-coded individually. Image courtesy of Dr. Vinothkumar.
How is cryo-EM single-particle analysis advantageous compared to traditional structural biology techniques?
Cryo-EM single-particle analysis brings unprecedented levels of insight to biomolecular research. Now structural biologists can study proteins and viruses and see how biological relevant forms interact with other macromolecules to show the basis of every biological process—for example, the mechanism by which a virus enters into a cell. Cryo-EM can also offer information about how a drug candidate binds to its target protein and very easily provide an image of the drug protein complex.
How is Thermo Fisher helping your cryo-EM research?
The Krios cryo-transmission electron microscope is highly automated. You can load up to 12 specimens and look at one at any given time. You can also set up data collection, go away, and when you come back you have enough data to process the next step, which is again automated. The full workflow created by Thermo Fisher has become so easy that anybody can use it. If you have seen the movie Ratatouille, where it’s said that “anybody can cook,” the same thing applies here. Now, because of the automation, anybody can do electron microscopy if they have a good specimen and good biological questioning.
What is your outlook on cryo-EM as a technique to combat diseases?
My outlook on the impact of cryo-EM on health and diseases can be explained with the example of the Zika virus outbreak in Brazil where lot of children got infected and died. Multiple groups of scientists took that as a challenge to use cryo-EM to obtain a structure of the Zika virus—and that structure helped to develop antibodies or small molecules and vaccines. It’s a good example for how cryo-EM has helped to tackle a disease. Also, on the Indian subcontinent, where we have a lot of tropical diseases like malaria, chikungunya, and dengue virus, cryo-EM could be the key to developing the next-generation of vaccines and antibodies that neutralize those diseases.
Steve Reyntjens is director of product marketing at Thermo Fisher Scientific.
To learn about how cryo-EM is helping to advance life sciences, fill out this form to speak with an expert.
Subscribe now to receive Accelerating Microscopy updates straight to your inbox.




Leave a Reply