Benefits of FBDD in the search for novel therapeutics
Fragment-based drug discovery (FBDD) is a method that screens a large quantity of protein fragments for their potential as small molecule therapeutics. This approach was previously hampered by the slow throughput of crystallization for the characterization of potential leads. The increasing accessibility of cryo-electron microscopy (cryo-EM) has addressed this challenge by providing high-throughput, near-atomic-resolution structural analysis along with automated data processing.
With cryo-EM, fragments can be screened for complex or flexible targets that were once considered undruggable. This blog explores how FBDD is actively being leveraged by researchers and companies such as Astex Pharmaceuticals, which is using this approach to accelerate lead discovery and expand the druggable proteome.
Approved oncology therapeutics leveraging fragment based drug discovery
In 2011, vemurafenib, developed by Plexxikon and Roche, was approved as the first small-molecule drug obtained from a fragment-based drug discovery campaign. As of today, seven small molecule drugs derived from fragment originators have been approved for oncology-related severe diseases.1
Fragment-based structural screening systematically converts weak, high-efficiency binders into potent, selective leads through a combination of structure-guided growth, linkage, and merger strategies. Compared with traditional high-throughput screening, fragment-based drug discovery campaigns sample the chemical space more economically, enrich this space for synthetically tractable hits, and provide early structural information, thereby lowering the overall risk of the screening optimization strategy. This ultimately reduces screening costs and accelerates the drug development process.
Since the approval of the first fragment-based drug, multiple companies have sought to leverage the FBDD approach, including Astex Pharmaceuticals. As a pioneer in fragment-based drug discovery, Astex has used structure-enabled medicinal chemistry to create oncology-related small-molecule therapeutics, including two FDA-approved, fragment-derived anticancer drugs: erdafitinib (created together with Janssen) and capivasertib (in collaboration with AstraZeneca).
While Astex initially utilized X-ray crystallography for FBDD, they have invested in cryo-EM as a complementary technology that can visualize challenging drug targets, such as membrane proteins. They have published a robust, fragment-based drug discovery workflow that utilizes cryo-EM to expand the druggable space and accelerate drug pipelines.
No need for crystals: cryo-EM unlocks fragment-based drug discovery campaigns
Single particle analysis is a cryo-EM technique that is able to generate high-resolution protein structures by imaging multiple instances of a sample at different angles, and then recombining these snapshots into a 3D representation. As a proof of concept, Astex Pharmaceuticals used single particle analysis on a β-galactosidase and the oncology drug target PKM2, showing that this technique is capable of routinely delivering 2.2 to 3.2 Å maps of the complexes. Even the small, chiral fragment ligands (which are around 150 Da) can be placed unambiguously in the electron density maps along with bound waters, alternative loop conformations, and sidechain rotamers.2 This level of detail is critical for medicinal chemistry cycles, and had previously represented a bottleneck in cryo-EM-based small-molecule drug discovery.
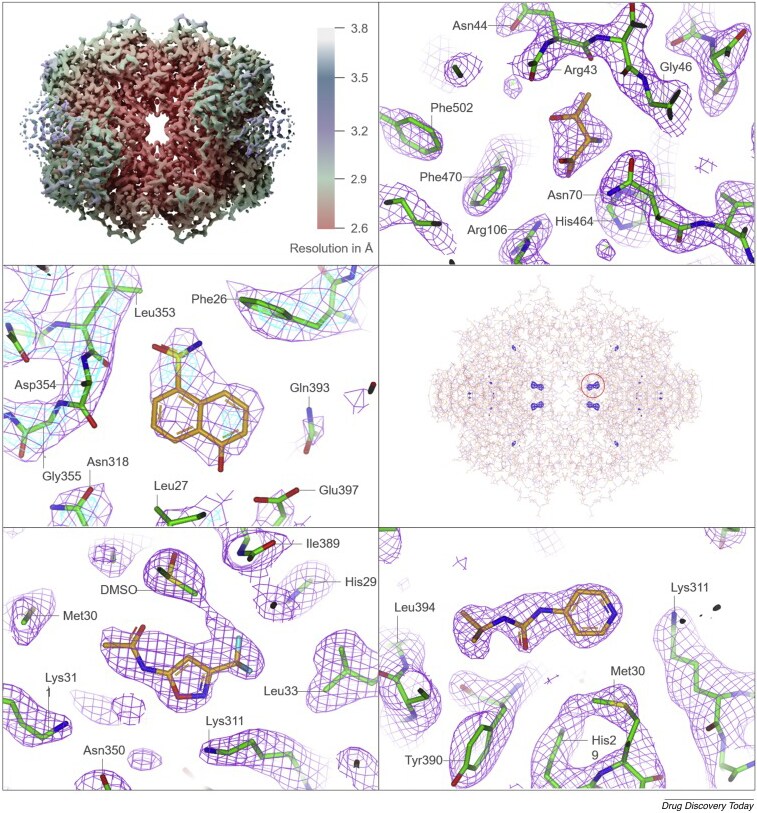
Figure 1. Fragment-based drug discovery screening of different small molecule fragments against β-galactosidase and the oncology drug target PKM2. Figure adapted from Saur et al. under CC BY 4.0.
Critically, this approach removed the need for time-consuming crystallization, which was necessary for structural analysis with X-ray crystallography. This also broadens the types of drug targets that can be structurally analyzed, enabling FBDD of difficult to crystallize samples, such as those containing flexible domains or different conformational states.
Additionally, their workflow integrates automated data processing and ligand fitting, which reduces subjective model interpretation and enables the parallel processing of tens of samples, including fragment cocktails, within practical fragment screening timelines. (The use of cocktails is critical for scaling FBDD to library sizes.)
This study also demonstrates that fragments can remain detectable even when the global resolution falls to 3 Å; this is because relevant binding pockets are reconstructed at higher local resolution and visualized with difference maps. Astex notes that they expect cryo-EM to evolve even further as a high-throughput tool for fragment-based drug discovery thanks to continuous improvements in the technique’s grid preparation, detector speed, and data acquisition capabilities.
Freezing a sliding target for fragment-based hit compound identification
Structure-based drug design targeting HIV focuses on transient, catalytically critical conformations that present ligandable sites resistant to viral escape. A study, spearheaded by researchers at the Rega Institute for Medical Research, Belgium, used single particle analysis to reveal and exploit a transient “P-pocket” in HIV-1 reverse transcriptase (RT), enabling fragment-guided optimization in near-native conditions.3
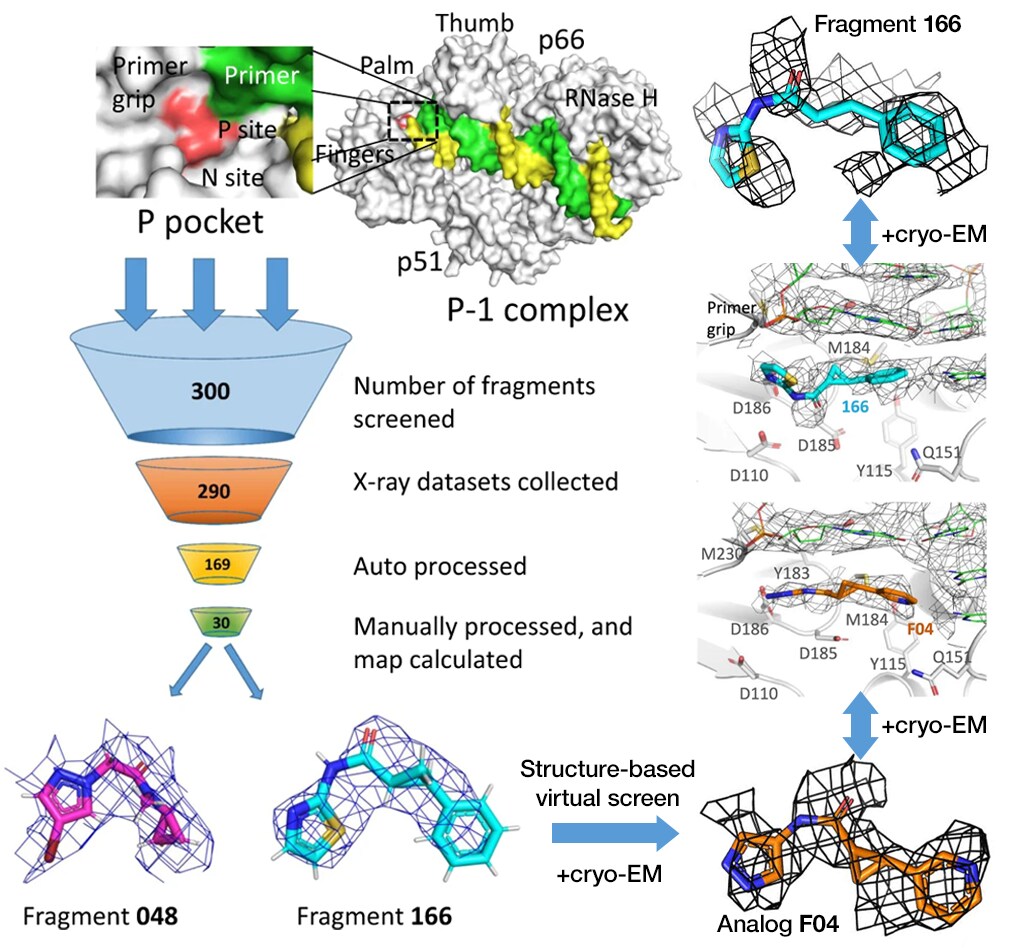
Figure 2. Fragment screening protocol that combines the complementary information of X-ray crystallography and cryo-EM. Figure adapted from Singh et al. under CC BY 4.0.
Initial crystallographic fragment screening of the P-pocket was limited by cadmium-dependent lattice stabilization and soak-induced crystal degradation, which led to P-pocket distortion and poor throughput. Cryo-EM circumvents these artifacts by trapping the P-1 state with a high-affinity DNA aptamer, allowing for the direct determination of the ligand-bound structures for fragment 166 and its analog F04. This strategy preserves the native pocket geometry and accelerates the FBDD process by removing the rate-limiting crystallization step.
Despite a global resolution between 3.38 and 3.58 Å, high local resolution was obtained within the P-pocket, providing confident assessment of fragment orientation and structure. This study highlights the utility of cryo-EM as a front-line structural biology technique for the FBDD of dynamic and nucleic-acid-coupled drug targets, such as viral polymerases.
Membrane protein drug targets that benefit from fragment-based drug discovery
Membrane proteins have, historically, been highly difficult to crystallize, and the resulting lack of high-resolution information has served as a bottleneck for fragment-based structural screening campaigns.
Astex Pharmaceuticals has recently published a robust, reliable, and high-throughput cryo-EM workflow for such membrane protein targets.4 Their approach was able to generate ten ligand-bound structures of various fragment-like agonists and antagonists at 2.1–2.4 Å resolution, linking binding modes to the open and closed pore conformations of the TRPML1 ion channel. The workflow emphasizes the practical requirements of fragment-based drug discovery: reproducibility, automation, rapid data handling, and consistent high-resolution structural determination.
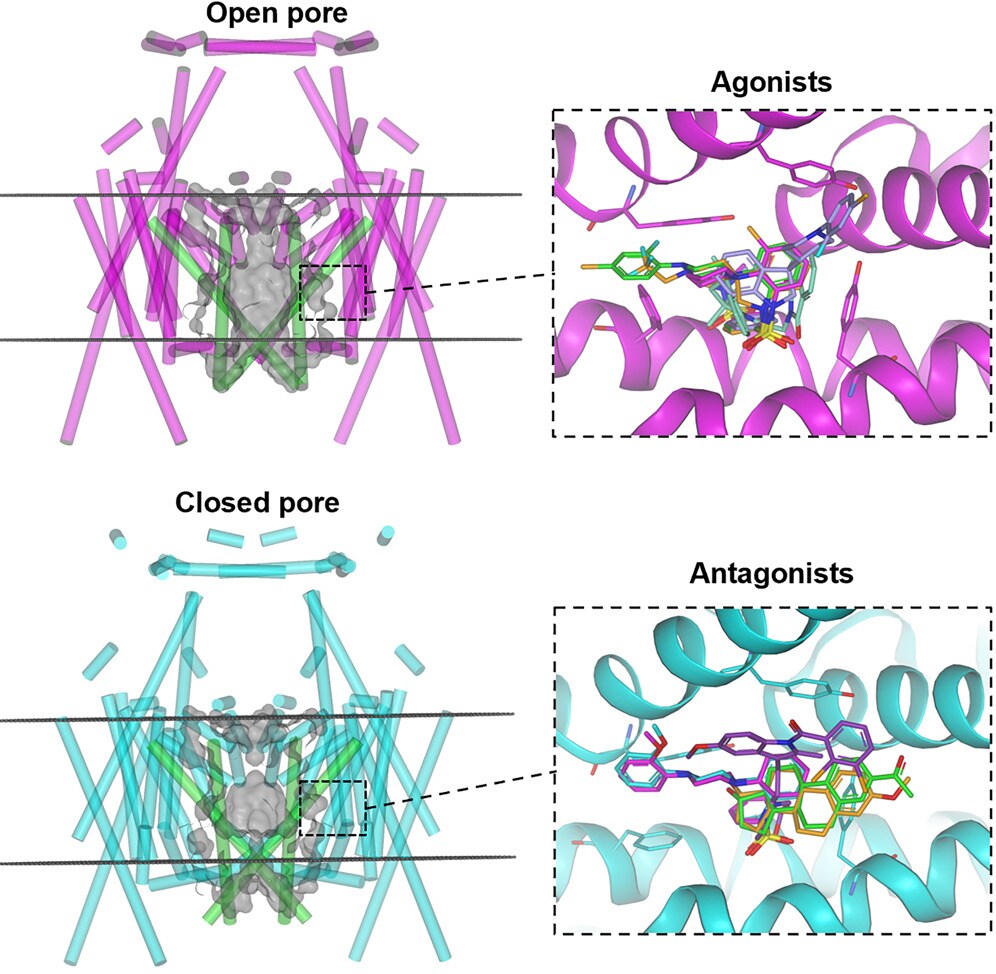
Figure 3. The cryo-EM structure of the TRPML1 ion channel, together with fragment-like modulator molecules, provides detailed high-resolution information to support iterative medicinal chemistry cycles. Figure adapted from Reeks et al. under CC BY 4.0.
The development of this standardized and robust workflow resulted in shorter cycle times for structure generation, typically requiring less than a week from initial request to fully refined structure.
Fragment-based drug discovery campaigns ramp up clinical pipelines
Fragment-based drug discovery has moved small molecule drugs from millimolar affinity fragments to marketed drugs that target various diseases. Cryo-EM supports these workflows with native-state electron density maps that can be used to consistently determine high-resolution, localized structures of ligand sites. This improves pose confidence and provides clear information on structure-activity relationships. The combination of automated workflows and unbiased ligand fitting generate reproducible conditions with high throughput, accelerating drug development cycles.
Cryo-EM data drives selectivity and supports earlier/better decision making, including for challenging targets such as membrane protein; this supports early pocket validation and brings near-atomic co-structures into medicinal chemistry cycles. These advancements broaden the druggable target space and reduce late-stage attrition through higher quality structural information, ultimately resulting in better decision-making, tighter structure-activity relationship information, and shorter pathways from fragments to differentiated drug candidates.
References
- Khedkar NR, et al. Fragment-Based Drug Discovery: Small Fragments, Big Impact – Success Stories of Approved Oncology Therapeutics. Bioorganic Chemistry 156 (2025). doi: 10.1016/j.bioorg.2025.108197
- Saur M, et al. Fragment-based drug discovery using cryo-EM. Drug Discovery Today 25:3 (2020). doi: 10.1016/j.drudis.2019.12.006
- Singh AK, et al. Sliding of HIV-1 reverse transcriptase over DNA creates a transient P pocket – targeting P-pocket by fragment screening. Nature Communications 12:7172 (2021). doi: 10.1038/s41467-021-27409-y
- Reeks J, et al. High throughput cryo-EM provides structural understanding for modulators of the lysosomal ion channel TRPML1. Structure 33:8 (2025). doi: 10.1016/j.str.2025.05.014
Learn more about small molecule drug discovery with electron microscopy >
Leave a Reply