For readers outside of the U.S., this content can be used as a case study resource.
While establishing your own laboratory-developed tests (LDTs) can offer certain advantages, the challenges associated with the process can seem daunting. How does one prepare to design and implement an LDT? What are the typical steps of LDT implementation? What is the typical LDT process and development timeline? To respond to such inquiries, Arizona State University College of Health Solutions has published an educational paper, which identifies key questions that must be addressed at each stage of the process.
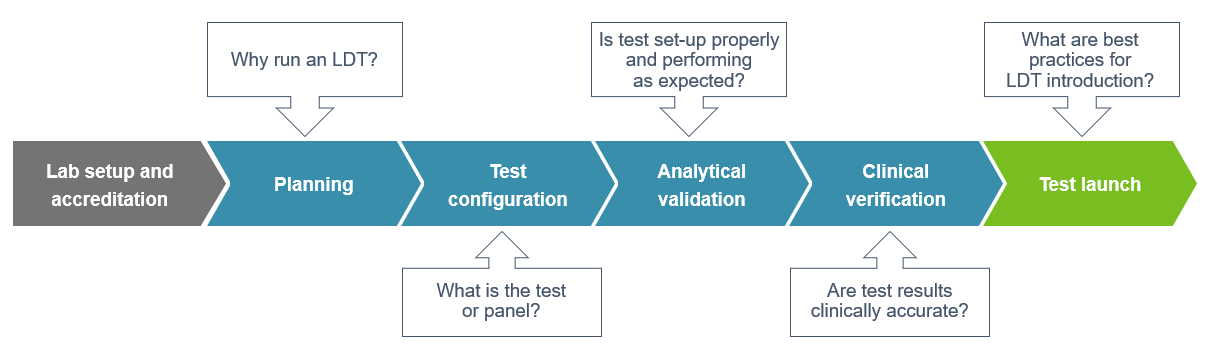
How are LDTs developed?
LDTs are often developed in response to a specific public health need when no (or limited) FDA-approved tests are available. This means the laboratories developing them must first be proficient in the technical aspects of the tests: specimen types, test methodology, range of detection, turnaround requirements, potential complementary tests, etc. LDTs might be as simple as adding a new analyte to an already established test, or as complex as launching a de novo multiplex panel. Additional considerations (new specimen types, instrumentation or biomarkers) add more layers of complexity to the development process. The research and development process must be structured and well-documented, with established Standard Operating Procedures (SOPs) and analytical validation studies which clearly show that the assay meets clinical requirements.
Preparing for and implementing a laboratory-developed test
- Licensure and certification: Testing laboratories must be certified under the Clinical Laboratory Improvement Amendments (CLIA) and licensed in the state in which it operates. Approval criteria for LDTs in the United States vary from state to state, making it important to understand your state’s requirements.
- Quality management and documentation: A Quality Management System (QMS) specifies the processes, procedures, responsibilities, data management, and continuous improvement required for achieving quality objectives.
- Proficiency requirements: Proficiency Testing (PT) is the testing of unknown samples sent to a laboratory by an approved PT program. All CLIA laboratories are required to conduct routine proficiency testing or develop an equivalent method for evaluating test performance.
- Personnel training documentation: SOPs must be periodically reviewed and formally approved. Significant changes to these documents may require retraining as well as competency assessments for all testing personnel.
- Implementing an LDT: After technical and legal feasibility is evaluated, the laboratory director and clinical consultant should draft a development plan with key clinicians to determine the assay’s design, intended use, and performance specifications. A freedom to operate analysis should assess the patent landscape for any potential patent infringement issues.
- Feasibility and design assessment: In the planning phase, potential drivers for developing the test should be examined. Considerations must be made regarding laboratory operations, in terms of the space, airflow, and technological training required. Analytical validation must be conducted to satisfy requirements for accuracy, sensitivity, specificity, and reproducibility.
- The path from development to launch: Overall, the process requires project planning, good documentation practices, quality control, analytical validation, and clinical verification. Depending on the technology, it can take up to 12 months to develop and validate an LDT for clinical use. Due to limited technical resources, it is typically the analytical validation period which is quite variable and often takes longer.
Consulting services for analytical validation are available to help laboratories complete their validation process up to 75% faster than typical laboratory-managed validations.
—
Arizona State University College of Health Solutions is dedicated to translating scientific health research and discovery into practical interventions. Be sure to read ASU’s educational paper for more information on preparing for and implementing a laboratory-developed test.
Don’t miss out on these related and informative sources:
Webinars:
- The Simple, Sensible, Salient & Still Spell-Binding Seven Questions About Laboratory Developed Tests
- Challenges of Establishing Laboratory-Developed Tests
Educational Papers:
- An introduction to diagnostic testing in laboratories
- Regulatory guidance for laboratories that design and implement diagnostic tests for clinical use
- Reimbursement for laboratory-developed and in vitro diagnostic tests
- Intellectual property associated with laboratory-developed tests (LDTs) and in vitro diagnostic (IVD) tests