Search Thermo Fisher Scientific
Thermo Scientific Chemicals
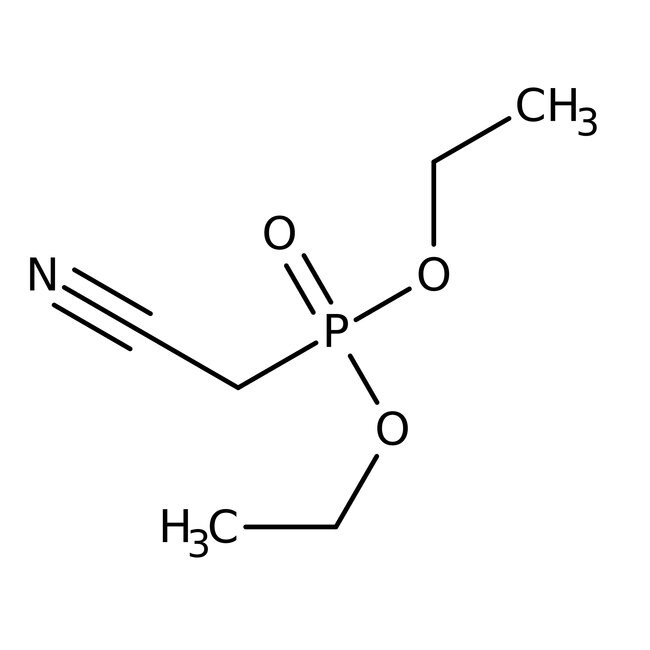
Diethyl cyanomethylphosphonate, 96%, Thermo Scientific Chemicals
Catalog number: A10218.22
100 g, Each

Thermo Scientific Chemicals
Diethyl cyanomethylphosphonate, 96%, Thermo Scientific Chemicals
Catalog number: A10218.22
100 g, Each
Quantity
Catalog number: A10218.22
also known as A10218-22
Price (USD)
Price: 326.00
Online price: 277.65
Your price:
Quantity
-
Chemical Identifiers
CAS
2537-48-6
IUPAC Name
diethyl (cyanomethyl)phosphonate
Molecular Formula
C6H12NO3P
InChI Key
KWMBADTWRIGGGG-UHFFFAOYSA-N
SMILES
CCOP(=O)(CC#N)OCC
Specifications
Appearance (Color)
Clear colorless to pale yellow
Form
Liquid
Assay (GC)
≥95.0%
Refractive Index
1.4290-1.4350 @ 20?C
Description
Diethyl cyanomethyl phosphonate is used as an intermediate in Horner-Emmons reaction for the synthesis of substituted nitriles and their amide and heterocyclic derivatives. It is known as a modified Wittig reagent used in the preparation of alpha, beta-unsaturated nitriles from ketones or aldehydes like 3-hydroxy-3-methylbutanal. It reacts with epoxides and nitrones to prepare cyano-substituted cyclopropanes and aziridines respectively. It is actively involved in the synthesis of alpha-arylated alkanenitriles via reaction with aryl iodides in presence of CuI.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diethyl cyanomethyl phosphonate is used as an intermediate in Horner-Emmons reaction for the synthesis of substituted nitriles and their amide and heterocyclic derivatives. It is known as a modified Wittig reagent used in the preparation of alpha, beta-unsaturated nitriles from ketones or aldehydes like 3-hydroxy-3-methylbutanal. It reacts with epoxides and nitrones to prepare cyano-substituted cyclopropanes and aziridines respectively. It is actively involved in the synthesis of alpha-arylated alkanenitriles via reaction with aryl iodides in presence of CuI.
Solubility
Miscible with water, chloroform, terahydrofuran, ethyl acetate and methylene chloride.
Notes
Incompatible with acids, bases, oxidizing agents and reducing agents.
Diethyl cyanomethyl phosphonate is used as an intermediate in Horner-Emmons reaction for the synthesis of substituted nitriles and their amide and heterocyclic derivatives. It is known as a modified Wittig reagent used in the preparation of alpha, beta-unsaturated nitriles from ketones or aldehydes like 3-hydroxy-3-methylbutanal. It reacts with epoxides and nitrones to prepare cyano-substituted cyclopropanes and aziridines respectively. It is actively involved in the synthesis of alpha-arylated alkanenitriles via reaction with aryl iodides in presence of CuI.
Solubility
Miscible with water, chloroform, terahydrofuran, ethyl acetate and methylene chloride.
Notes
Incompatible with acids, bases, oxidizing agents and reducing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text