Search Thermo Fisher Scientific
Ethyl potassium malonate, 98%, Thermo Scientific Chemicals
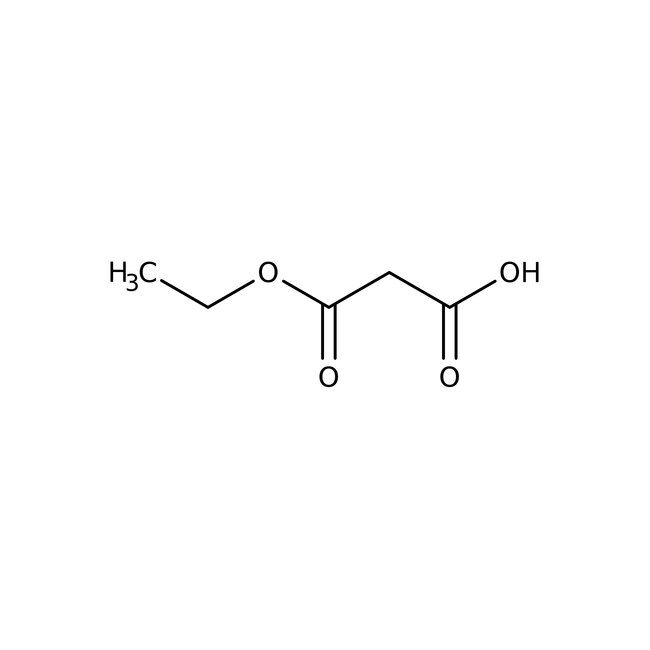
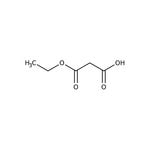

Ethyl potassium malonate, 98%, Thermo Scientific Chemicals
Chemical Identifiers
Specifications
Description
Ethyl potassium malonate is used as a competitive inhibitor of the enzyme succinate dehydrogenase. It acts as a precursor to produce (trimethylsilyl)ethyl malonate, which is utilized to prepare beta-ketoesters by acylation. Further, it reacts with aryl nitriles to prepare beta-amino acrylates in the presence of zinc chloride and a catalytic amount of Hünig's base. In addition to this, it serves as an intermediate for the preparation of ethyl tert-butyl malonate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Ethyl potassium malonate is used as a competitive inhibitor of the enzyme succinate dehydrogenase. It acts as a precursor to produce (trimethylsilyl)ethyl malonate, which is utilized to prepare beta-ketoesters by acylation. Further, it reacts with aryl nitriles to prepare beta-amino acrylates in the presence of zinc chloride and a catalytic amount of Hünig′s base. In addition to this, it serves as an intermediate for the preparation of ethyl tert-butyl malonate.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.