Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Sodium bis(2-methoxyethoxy)aluminum hydride, 70% w/w in toluene., Thermo Scientific Chemicals
Catalog number: A13292.0B
1000 g, Each


Thermo Scientific Chemicals
Sodium bis(2-methoxyethoxy)aluminum hydride, 70% w/w in toluene., Thermo Scientific Chemicals
Catalog number: A13292.0B
1000 g, Each
Quantity
Catalog number: A13292.0B
also known as A13292-0B
Price (USD)
Price: 219.00
Your price:
Quantity
-
Chemical Identifiers
CAS
22722-98-1
IUPAC Name
alumanylium sodium bis(2-methoxyethan-1-olate)
Molecular Formula
C6H16AlNaO4
InChI Key
XJIQVZMZXHEYOY-UHFFFAOYSA-N
SMILES
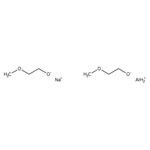
[Na+].[AlH2+].COCC[O-].COCC[O-]
Specifications
Form
Viscous liquid
Assay (unspecified)
≥68.0 to ≤72.0% (w/w)
Appearance (Color)
Clear or slightly turbid, colorless to pale orange-brown
Description
Sodium bis(2-methoxyethoxy)aluminum hydride acts as a reducing agent in organic synthesis. It is used to prepare azoxyarenes, azoarenes and hydroazoarenes from nitroarenes. It plays an important role for the conversion of aldehydes, ketones, carboxylic acids, esters, acyl halides and anhydrides to primary alcohols. It is also employed in hydroaluminate alkenes and alkynes. It is utilized in the reduction of lactones and epoxides into diols. Further, it serves as a methylation reagent for aryl-activated compounds. In addition to this, it is involved in the reduction of amides, nitriles and amines to the corresponding amines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium bis(2-methoxyethoxy)aluminum hydride acts as a reducing agent in organic synthesis. It is used to prepare azoxyarenes, azoarenes and hydroazoarenes from nitroarenes. It plays an important role for the conversion of aldehydes, ketones, carboxylic acids, esters, acyl halides and anhydrides to primary alcohols. It is also employed in hydroaluminate alkenes and alkynes. It is utilized in the reduction of lactones and epoxides into diols. Further, it serves as a methylation reagent for aryl-activated compounds. In addition to this, it is involved in the reduction of amides, nitriles and amines to the corresponding amines.
Solubility
Miscible with aromatic hydrocarbons, ether, tetrahydrofuran, dimethyl ether and dimethylformamide. Immiscible with aliphatic hydrocarbons.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with water, oxidizing agents and combustible material.
Sodium bis(2-methoxyethoxy)aluminum hydride acts as a reducing agent in organic synthesis. It is used to prepare azoxyarenes, azoarenes and hydroazoarenes from nitroarenes. It plays an important role for the conversion of aldehydes, ketones, carboxylic acids, esters, acyl halides and anhydrides to primary alcohols. It is also employed in hydroaluminate alkenes and alkynes. It is utilized in the reduction of lactones and epoxides into diols. Further, it serves as a methylation reagent for aryl-activated compounds. In addition to this, it is involved in the reduction of amides, nitriles and amines to the corresponding amines.
Solubility
Miscible with aromatic hydrocarbons, ether, tetrahydrofuran, dimethyl ether and dimethylformamide. Immiscible with aliphatic hydrocarbons.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with water, oxidizing agents and combustible material.
WARNING: Reproductive Harm - www.P65Warnings.ca.gov
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text