Search
Thermo Scientific Chemicals
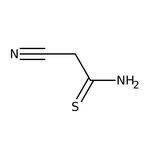
2-Cyanothioacetamide, 98%
CAS: 7357-70-2 | C3H4N2S | 100.14 g/mol
Catalog number A15436.14
also known as A15436-14
Price (TWD)Request A Quote
-
Quantity:
25 g
Chemical Identifiers
CAS7357-70-2
IUPAC Name2-cyanoethanethioamide
Molecular FormulaC3H4N2S
InChI KeyBHPYMZQTCPRLNR-UHFFFAOYSA-N
SMILESNC(=S)CC#N
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥97.5%
Identification (FTIR)Conforms
Melting Point (clear melt)110-120°C
Appearance (Color)Yellow-orange to brown
FormCrystals or powder or crystalline powder
2-Cyanothioacetamide was used in the synthesis of 3,4-trans-4-aryl-3-(1-pyridinio)-1,2,3,4-tetrahydropyridine-6-thiolates and bis[6-(2-aryl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile]. 2-Cyanothioacetamide is used as a building block for 2-pyridothiones. It can be used in agrochemical, pharmaceutical and dyestuff field etc,.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Cyanothioacetamide was used in the synthesis of 3,4-trans-4-aryl-3-(1-pyridinio)-1,2,3,4-tetrahydropyridine-6-thiolates and bis[6-(2-aryl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile]. 2-Cyanothioacetamide is used as a building block for 2-pyridothiones. It can be used in agrochemical, pharmaceutical and dyestuff field etc,.
Solubility
Does not mix well with water.
Notes
Light Sensitive. Store in the dark.
2-Cyanothioacetamide was used in the synthesis of 3,4-trans-4-aryl-3-(1-pyridinio)-1,2,3,4-tetrahydropyridine-6-thiolates and bis[6-(2-aryl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile]. 2-Cyanothioacetamide is used as a building block for 2-pyridothiones. It can be used in agrochemical, pharmaceutical and dyestuff field etc,.
Solubility
Does not mix well with water.
Notes
Light Sensitive. Store in the dark.
RUO – Research Use Only
General References:
- Elgemeie G. E. H. ; Ali H . A. ; Eid M. M. Reaction of 2-cyanothioacetamide with α-alkylated β-diketones: synthesis of pyridine-2(1H)-thione, thieno[2,3-b]pyridine and pyrido[2,3-c]pyrazole derivatives. Journal of Chemical Research. Synopses.1993, 7 256-257.
- Cyclocondensation with Benzoyl isothiocyanate, L06964 results in the formation of a pyrimidinedithiol: Chem. Ind. (London), 270 (1988):
- See also Methyl isothiocyanate, A11757.
- For a review on the utility of cyanothioacetamide in heterocyclic synthesis, see: Heterocycles, 24, 2023 (1986).