Search Thermo Fisher Scientific
- Contact Us
- Quick Order
-
Don't have an account ? Create Account
Search Thermo Fisher Scientific
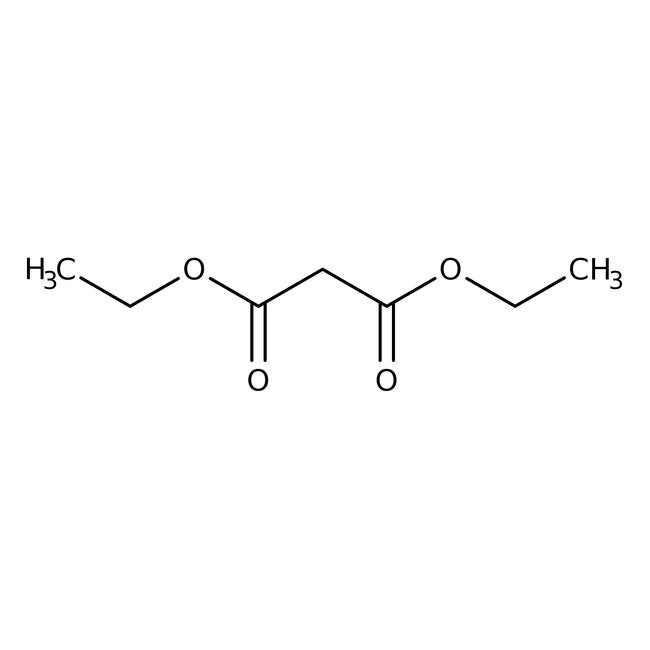

This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.