Search
Thermo Scientific Chemicals
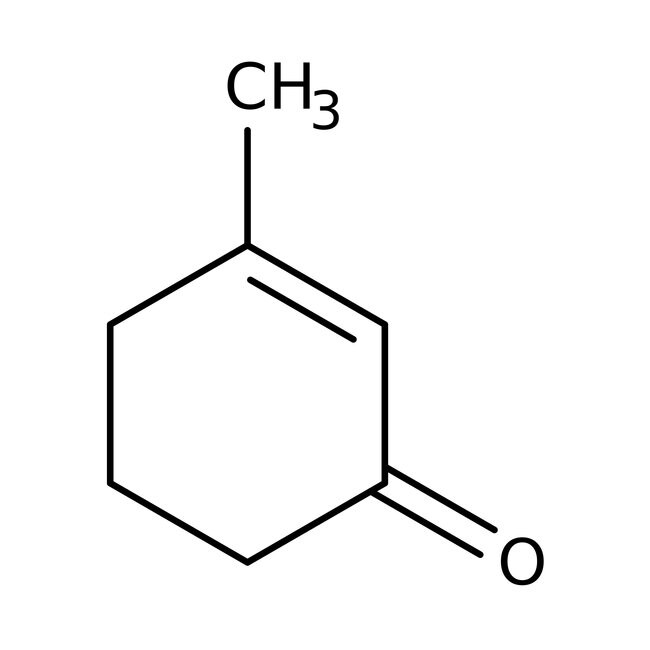
3-Methyl-2-cyclohexen-1-one, 98%
CAS: 1193-18-6 | C7H10O | 110.16 g/mol
Catalog number A15704.14
also known as A15704-14
Price (TWD)Request A Quote
-
Quantity:
25 g
Chemical Identifiers
CAS1193-18-6
IUPAC Name3-methylcyclohex-2-en-1-one
Molecular FormulaC7H10O
InChI KeyIITQJMYAYSNIMI-UHFFFAOYSA-N
SMILESCC1=CC(=O)CCC1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
Assay (GC)≥97.5%
FormLiquid
Refractive Index1.4930-1.4970 @ 20?C
3-Methyl-2-cyclohexen-1-one was used in the synthesis of 19-nor-1α, 25-dihydroxyvitamin D(3) derivatives. It was also used in sex pheromone of the Douglas-fir beetle. Used in nut flavor.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Methyl-2-cyclohexen-1-one was used in the synthesis of 19-nor-1α, 25-dihydroxyvitamin D(3) derivatives. It was also used in sex pheromone of the Douglas-fir beetle. Used in nut flavor.
Solubility
Soluble in alcohol. Insoluble in water.
Notes
Store in cool, well ventilated area. Keep container tightly closed. Store away form oxidizing agent.
3-Methyl-2-cyclohexen-1-one was used in the synthesis of 19-nor-1α, 25-dihydroxyvitamin D(3) derivatives. It was also used in sex pheromone of the Douglas-fir beetle. Used in nut flavor.
Solubility
Soluble in alcohol. Insoluble in water.
Notes
Store in cool, well ventilated area. Keep container tightly closed. Store away form oxidizing agent.
RUO – Research Use Only
General References:
- X Zhou; G D Zhu; D Van Haver; M Vandewalle; P J De Clercq; A Verstuyf; R Bouillon. Synthesis, biological activity, and conformational analysis of four seco-D-15,19-bisnor-1alpha,25-dihydroxyvitamin D analogues, diastereomeric at C17 and C20. Journal of Medicinal Chemistry. 1999, 42 (18), 3539-3556.
- Donald S. Noyce, Malcolm. Evett. Mechanism of the acid-catalyzed double bond migration in 3-cyclohexen-1-one and 3-methyl-3-cyclohexen-1-one. J. Org. Chem. 1972, 37 (3), 394-397.
- For photochemical (2 + 2) photocycloaddition reaction with ethylene, as a general method for synthesis of fused cyclobutane systems, see: Org. Synth. Coll., 7, 315 (1990).
- For conversion to the silyl enol ether and stereoselective aldol-type coupling, see: Tetrahedron, 45, 6101 (1989):