Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Strontium chloride hexahydrate, 98%, Thermo Scientific Chemicals
Catalog number: A16764.30
250 g, Each


Thermo Scientific Chemicals
Strontium chloride hexahydrate, 98%, Thermo Scientific Chemicals
Catalog number: A16764.30
250 g, Each
Quantity
Catalog number: A16764.30
also known as A16764-30
Price (USD)
Price: 63.40
Online price: 53.65
Your price:
Quantity
-
Chemical Identifiers
CAS
10025-70-4
IUPAC Name
strontium(2+) hexahydrate dichloride
Molecular Formula
Cl2H12O6Sr
InChI Key
AMGRXJSJSONEEG-UHFFFAOYSA-L
SMILES
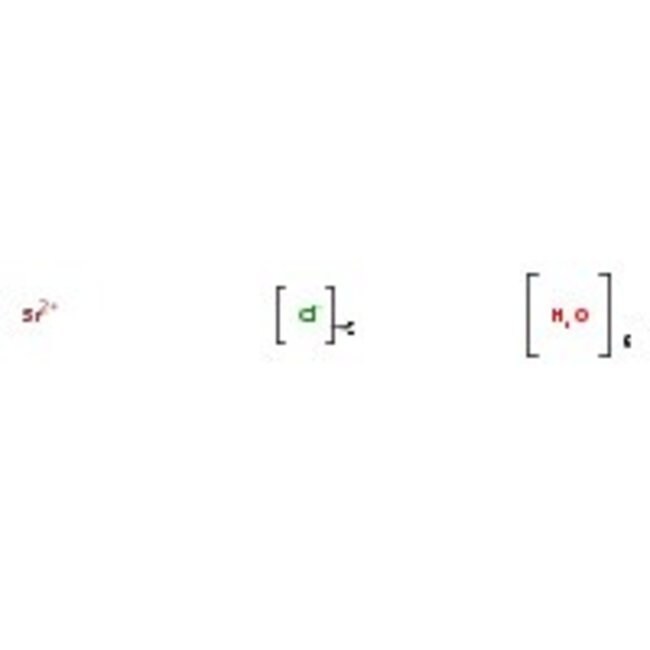
O.O.O.O.O.O.[Cl-].[Cl-].[Sr++]
Specifications
Appearance (Color)
White
Form
Crystals or powder or crystalline powder or lumps
Assay (Titration ex Chloride)
≥98.0 to ≤102.0%
Identification (FTIR)
Conforms
Water Content (Karl Fischer Titration)
38.00-42.90% (5.4-6.6 waters)
Description
Strontium chloride hexahydrate is a precursor to prepare strontium chromate which is used as a corrosion inhibitor for aluminum. It is involved in the synthesis of cadmium sulfide core photocatalytic nanoparticles. It is used as a red coloring agent in pyrotechnics. Furthermore, it is utilized in glass-making and metallurgy. In the medical field, its radioactive isotope strontium-89 is used for the treatment of bone cancer. It acts as a catalyst in the conversion of ketones and aldehydes to the corresponding gem-dihydroperoxides using aqueous hydrogen peroxide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Strontium chloride hexahydrate is a precursor to prepare strontium chromate which is used as a corrosion inhibitor for aluminum. It is involved in the synthesis of cadmium sulfide core photocatalytic nanoparticles. It is used as a red coloring agent in pyrotechnics. Furthermore, it is utilized in glass-making and metallurgy. In the medical field, its radioactive isotope strontium-89 is used for the treatment of bone cancer. It acts as a catalyst in the conversion of ketones and aldehydes to the corresponding gem-dihydroperoxides using aqueous hydrogen peroxide.
Solubility
Soluble in water. Slightly soluble in ethanol and acetone. Insoluble in ammonia.
Notes
Incompatible with strong oxidizing agents.
Strontium chloride hexahydrate is a precursor to prepare strontium chromate which is used as a corrosion inhibitor for aluminum. It is involved in the synthesis of cadmium sulfide core photocatalytic nanoparticles. It is used as a red coloring agent in pyrotechnics. Furthermore, it is utilized in glass-making and metallurgy. In the medical field, its radioactive isotope strontium-89 is used for the treatment of bone cancer. It acts as a catalyst in the conversion of ketones and aldehydes to the corresponding gem-dihydroperoxides using aqueous hydrogen peroxide.
Solubility
Soluble in water. Slightly soluble in ethanol and acetone. Insoluble in ammonia.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text