Search Thermo Fisher Scientific
Thermo Scientific Chemicals
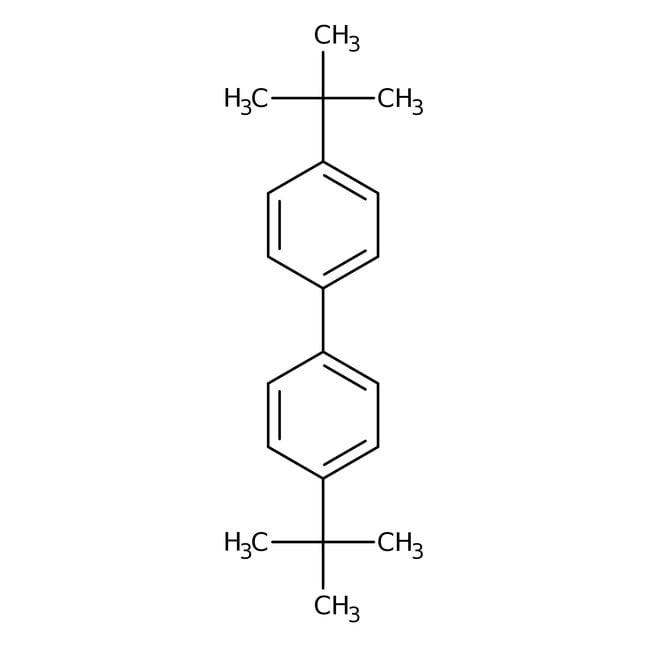
4,4'-Di-tert-butylbiphenyl, 99%, Thermo Scientific Chemicals
Catalog number: B21470.14
25 g, Each


Thermo Scientific Chemicals
4,4'-Di-tert-butylbiphenyl, 99%, Thermo Scientific Chemicals
Catalog number: B21470.14
25 g, Each
Quantity
Catalog number: B21470.14
also known as B21470-14
Price (USD)
Price: 156.00
Online price: 135.65
Your price:
Quantity
-
Chemical Identifiers
CAS
1625-91-8
IUPAC Name
4,4'-di-tert-butyl-1,1'-biphenyl
Molecular Formula
C20H26
InChI Key
CDKCEZNPAYWORX-UHFFFAOYSA-N
SMILES
CC(C)(C)C1=CC=C(C=C1)C1=CC=C(C=C1)C(C)(C)C
Specifications
Appearance (Color)
White to pale yellow
Form
Crystals or powder or crystalline powder
Assay (GC)
≥98.5%
Melting Point (clear melt)
124.0-131.0?C
Description
It is used in the generation of 1,2-di(lithiomethyl)benzene. It is found to accept electrons from Li metal to give a radical anion which is highly effective in the conversion of alkyl halides to alkyllithiums. 4,4?-Di-tert-butylbiphenyl is used in production of homoallylic amine derivatives. It is also used in the preparation of lithium di-tert-butylbiphenylide, a radical anion, superior to sodium or lithium naphthalenides for metalation reactions. Along with lithium, 4,4?-Di-tert-butylbiphenyl catalyzes; reaction of chloromethyl ethyl ether and different carbonyl compounds to yield corresponding hydroxyethers and reductive opening of N-phenylazetidine.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used in the generation of 1,2-di(lithiomethyl)benzene. It is found to accept electrons from Li metal to give a radical anion which is highly effective in the conversion of alkyl halides to alkyllithiums. 4,4′-Di-tert-butylbiphenyl is used in production of homoallylic amine derivatives. It is also used in the preparation of lithium di-tert-butylbiphenylide, a radical anion, superior to sodium or lithium naphthalenides for metalation reactions. Along with lithium, 4,4′-Di-tert-butylbiphenyl catalyzes; reaction of chloromethyl ethyl ether and different carbonyl compounds to yield corresponding hydroxyethers and reductive opening of N-phenylazetidine.
Solubility
Solubility in toluene; almost transparency. Soluble in dioxane: 0.1 g/mL, (clear).
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It is used in the generation of 1,2-di(lithiomethyl)benzene. It is found to accept electrons from Li metal to give a radical anion which is highly effective in the conversion of alkyl halides to alkyllithiums. 4,4′-Di-tert-butylbiphenyl is used in production of homoallylic amine derivatives. It is also used in the preparation of lithium di-tert-butylbiphenylide, a radical anion, superior to sodium or lithium naphthalenides for metalation reactions. Along with lithium, 4,4′-Di-tert-butylbiphenyl catalyzes; reaction of chloromethyl ethyl ether and different carbonyl compounds to yield corresponding hydroxyethers and reductive opening of N-phenylazetidine.
Solubility
Solubility in toluene; almost transparency. Soluble in dioxane: 0.1 g/mL, (clear).
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text