Search
Thermo Scientific Chemicals
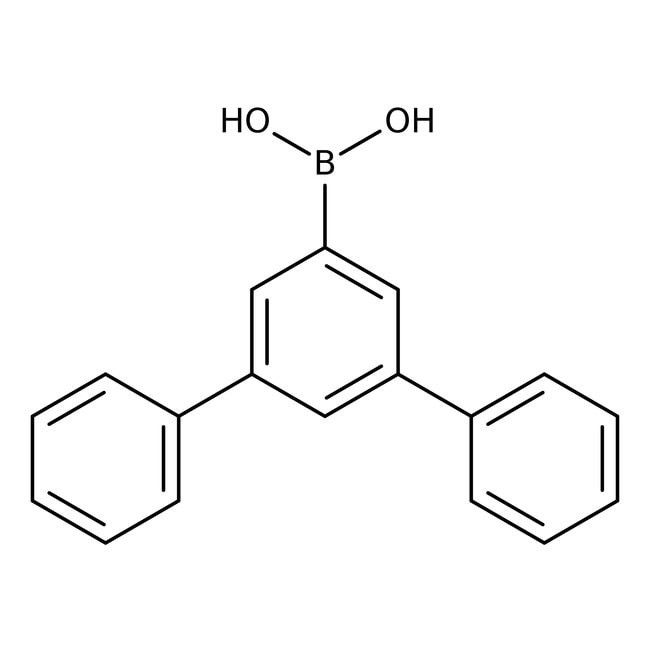
1,1':3',1″-Terphenyl-5'-boronic acid, 95%
CAS: 128388-54-5 | C18H15BO2 | 274.126 g/mol
Catalog number H27640.03
also known as H27640-03
Price (TWD)Request A Quote
-
Quantity:
1 g
Chemical Identifiers
CAS128388-54-5
IUPAC Name{5-phenyl-[1,1'-biphenyl]-3-yl}boronic acid
Molecular FormulaC18H15BO2
InChI KeyMRBZYVMZUBUDAX-UHFFFAOYSA-N
SMILESOB(O)C1=CC(=CC(=C1)C1=CC=CC=C1)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
FormPowder
Assay (Aqueous acid-base Titration)≥94.0%
Assay (HPLC)≥94.0%
Proton NMRConforms to structure
View more
A double Suzuki cross-coupling protocol has been devised as a practical route to a variety of terphenyls. Good chemoselectivity was observed. Unsymmetrically substituted triphenylenes were also easily prepared. A double Suzuki cross-coupling protocol has been devised as a practical route to a variety of terphenyls and triphenylenes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
A double Suzuki cross-coupling protocol has been devised as a practical route to a variety of terphenyls. Good chemoselectivity was observed. Unsymmetrically substituted triphenylenes were also easily prepared. A double Suzuki cross-coupling protocol has been devised as a practical route to a variety of terphenyls and triphenylenes.
Solubility
Insoluble in water.
Notes
Refrigerated (2-4°C). Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
A double Suzuki cross-coupling protocol has been devised as a practical route to a variety of terphenyls. Good chemoselectivity was observed. Unsymmetrically substituted triphenylenes were also easily prepared. A double Suzuki cross-coupling protocol has been devised as a practical route to a variety of terphenyls and triphenylenes.
Solubility
Insoluble in water.
Notes
Refrigerated (2-4°C). Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
General References:
- M.J. Sharp,; W. Cheng,; V. Snieckus. Synthetic connections to the aromatic directed metalation reaction. Functionalized aryl boronic acids by ipso borodesilylation. General syntheses of unsymmetrical biphenyls and m-terphenyls. Tetrahedron Letters. 1987, 28 (43),5093-5096.
- Daniele Simonia,; Giuseppe Gianninib,; Pier Giovanni Baraldia,; Romeo Romagnolia,; Marinella Robertic,; Riccardo Rondanina,; Riccardo Baruchelloa,; Giuseppina Grisoliaa,; Marcello Rossia,; Danilo Mirizzia,; Francesco Paolo Invidiatad,; Stefania Grimaudoe,; Manlio Tolomeo. A convenient synthesis of unsymmetrically substituted terphenyls of biologically active stilbenes via a double Suzuki cross-coupling protocol. Tetrahedron Letters. 2003, 44 (14), 3005-3008.