Search
Thermo Scientific Chemicals
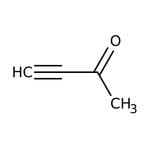
3-Butin-2-ona, 98 %, Thermo Scientific Chemicals
CAS: 1423-60-5 | C4H4O | 68.08 g/mol
Número de catálogo L05527.14
también denominado L05527-14
Precio (USD)
-
Cantidad:
25 g
Identificadores químicos
CAS1423-60-5
Especificaciones Hoja de especificaciones
Hoja de especificaciones
Appearance (Color)Clear colorless to yellow
FormLiquid
Assay (GC)≥96.0%
Identification (FTIR)Conforms
Refractive Index1.4045-1.4085 @ 20°C
3-Butyn-2-one was used in the synthesis of clerodane diterpenoid (±)-sacacarin. It was used as substrate in stereoselective, conjugate arylation mediated by gallium(III) chloride leading to (E)-α,β-unsaturated ketones. 3-Butyn-2-one undergoes asymmetric double-Michael reaction with ortho-tosylamidophenyl malonate catalyzed by chiral aminophosphines to yield indolines2. It undergoes double Michael reaction with nitrogen-containing tethered diacid to give pipecolic acid derivatives. 3-Butyn-2-one, is used as a reactant with Dimethyl acetone-1,3-dicarboxylate, under mild conditions to give a benzenoid product via a Michael addition - aldol cyclization process.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Aplicaciones
La 3-butin-2-ona se utilizó una en la síntesis de diterpenoide clerodano (+/-)-sacarina. Se usó como sustrato en la arilación conjugada estereoselectiva mediada por cloruro de galio(III) que conduce a (E)-α,β-cetonas insaturadas. La 3-butin-2-ona se somete a una doble reacción asimétrica de Michael con malonato de ortotosilamidofenilo catalizado con aminofosfinas quirales para producir indolinas2. Pasa por una doble reacción de Michael con diácido unido que contiene nitrógeno para proporcionar derivados de ácido pipecólico. La 3-butin-2-ona se utiliza como reactivo con dimetilacetona-1,3-dicarboxilato en condiciones moderadas para proporcionar un producto bencenoide mediante un proceso de ciclización de adición de aldol de Michael.
Solubilidad
Es soluble en agua.
Notas
Estable bajo temperaturas y presiones normales. Almacenar en un lugar fresco. Mantener el recipiente bien cerrado en un lugar seco y bien ventilado. Mantener alejado de los agentes oxidantes fuertes.
La 3-butin-2-ona se utilizó una en la síntesis de diterpenoide clerodano (+/-)-sacarina. Se usó como sustrato en la arilación conjugada estereoselectiva mediada por cloruro de galio(III) que conduce a (E)-α,β-cetonas insaturadas. La 3-butin-2-ona se somete a una doble reacción asimétrica de Michael con malonato de ortotosilamidofenilo catalizado con aminofosfinas quirales para producir indolinas2. Pasa por una doble reacción de Michael con diácido unido que contiene nitrógeno para proporcionar derivados de ácido pipecólico. La 3-butin-2-ona se utiliza como reactivo con dimetilacetona-1,3-dicarboxilato en condiciones moderadas para proporcionar un producto bencenoide mediante un proceso de ciclización de adición de aldol de Michael.
Solubilidad
Es soluble en agua.
Notas
Estable bajo temperaturas y presiones normales. Almacenar en un lugar fresco. Mantener el recipiente bien cerrado en un lugar seco y bien ventilado. Mantener alejado de los agentes oxidantes fuertes.
RUO – Research Use Only
General References:
- H Monti.; G Audran.; G Léandri.; JP Monti. ZnI 2 Catalyzed [2+ 2] versus [3+ 2] cycloaddition of an allyltrimethylsilane with 3-butyn-2-one: Confirmation of a cyclobutene by-product formation. Tetrahedron letters. 1994, 35,(19), 3073-3076.
- IH Um.; EJ Lee.; JS Min. Remarkable catalytic effect of H+ in Michael-type additions of anilines to 3-butyn-2-one. Tetrahedron letters. 2001, 57,(47), 9585-9589.
- Reacts with Dimethyl acetone-1,3-dicarboxyl ate, A14969, under mild conditions to give a benzenoid product via a Michael addition - aldol cyclization process: Synth. Commun., 28, 1525 (1998):
- For a similar reaction, see Methyl 2-hexynoate, B23065.