Search
Thermo Scientific Chemicals
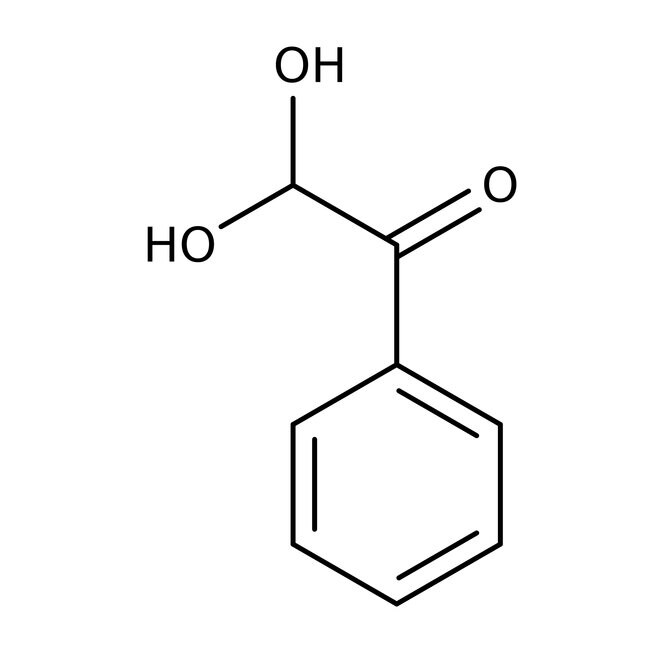
Fenilglioxal monohidrato, 97 %, Thermo Scientific Chemicals
CAS: 1075-06-5 | C8H8O3 | 152.149 g/mol
Número de catálogo A10677.22
también denominado A10677-22
Precio (USD)
-
Cantidad:
100 g
Identificadores químicos
CAS1075-06-5
IUPAC Name2,2-dihydroxy-1-phenylethan-1-one
Molecular FormulaC8H8O3
InChI KeyNBIBDIKAOBCFJN-UHFFFAOYSA-N
SMILESOC(O)C(=O)C1=CC=CC=C1
Ver más
Especificaciones Hoja de especificaciones
Hoja de especificaciones
FormCrystals or powder or crystalline powder
Identification (FTIR)Conforms
Water Content (Karl Fischer Titration)6.29-16.77% (0.5-1.5 waters)
Appearance (Color)White to cream to pale brown or pink
Assay (GC)≥96.0%
Phenylglyoxal monohydrate was used to modify pig muscle carbonic anhydraseIII and as a specific reagent for arginine groups. It was also used to prepare pyrrolinone and furan derivatives and as as a chemiluminescent reagent for the determination of purines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Aplicaciones
El monohidrato de fenilglioxal se utilizó para modificar la anhidrasa III carbónica muscular de cerdo y como reactivo específico para grupos de arginina. También se utilizó para preparar derivados de pirrolinona y furano y como reactivo quimioluminiscente para la determinación de purinas.
Solubilidad
Parcialmente miscible con agua.
Notas
Estables en las condiciones de almacenamiento recomendadas. Incompatible con agentes oxidantes.
El monohidrato de fenilglioxal se utilizó para modificar la anhidrasa III carbónica muscular de cerdo y como reactivo específico para grupos de arginina. También se utilizó para preparar derivados de pirrolinona y furano y como reactivo quimioluminiscente para la determinación de purinas.
Solubilidad
Parcialmente miscible con agua.
Notas
Estables en las condiciones de almacenamiento recomendadas. Incompatible con agentes oxidantes.
RUO – Research Use Only
General References:
- Masaaki Kai; Yosuke Ohkura; Sayuri Yonekura; Masatake Iwasaki. Selective determination of guanine and its nucleosides and nucleotides by reaction with phenylglyoxal as a fluorogenic reagent. Analytica Chimica Acta. 1988, 207, 243-249
- Undergoes the Lewis-acid-catalyzed ene-insertion reaction with simple alkenes. Periodate cleavage of the resulting ɑ-hydroxyketones has been used as a route to ß-unsaturated aldehydes: Synthesis, 413 (1987):