Search
Thermo Scientific Chemicals
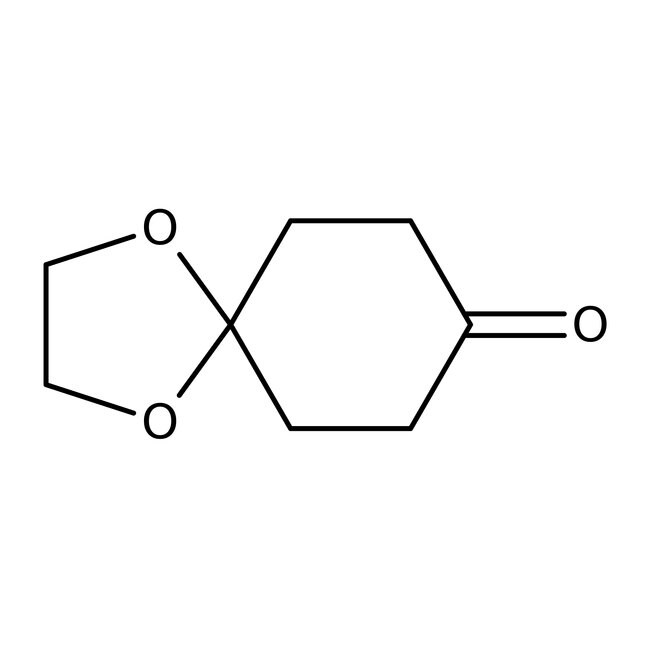
1,4-Cyclohexanedione monoethylene acetal, 97%
CAS: 4746-97-8 | C8H12O3 | 156.181 g/mol
Catalog number A10408.14
also known as A10408-14
Price (EUR)
108,00
Each
Quantity:
25 g
Price (EUR)
108,00
Each
Chemical Identifiers
CAS4746-97-8
IUPAC Name1,4-dioxaspiro[4.5]decan-8-one
Molecular FormulaC8H12O3
InChI KeyVKRKCBWIVLSRBJ-UHFFFAOYSA-N
SMILESO=C1CCC2(CC1)OCCO2
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale brown
Formcrystals or crystalline powder
Solution Test10% w/v in acetone : Clear
Assay (GC)> 96.0%
1,4-Cyclohexanedione is used in the preparation of series of potent analgesic compounds. 1,4-Cyclohexanedione is also used as a building block in the synthesis of tritium labelled probes for the autoradiography study of the dopamine reuptake complex.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,4-Cyclohexanedione is used in the preparation of series of potent analgesic compounds. 1,4-Cyclohexanedione is also used as a building block in the synthesis of tritium labelled probes for the autoradiography study of the dopamine reuptake complex.
Solubility
Soluble in chloroform & methanol.
Notes
Moisture Sensitive. Store away from moisture and oxidizing agents. Incompatible with strong oxidizing agents and strong acids. Ensure adequate ventilation during use.
1,4-Cyclohexanedione is used in the preparation of series of potent analgesic compounds. 1,4-Cyclohexanedione is also used as a building block in the synthesis of tritium labelled probes for the autoradiography study of the dopamine reuptake complex.
Solubility
Soluble in chloroform & methanol.
Notes
Moisture Sensitive. Store away from moisture and oxidizing agents. Incompatible with strong oxidizing agents and strong acids. Ensure adequate ventilation during use.
RUO – Research Use Only
General References:
- Chad T. Hamik; Niklas Manz; and Oliver Steinbock. Anomalous Dispersion and Attractive Pulse Interaction in the 1,4-Cyclohexanedione Belousov-Zhabotinsky Reaction. J. Phys. Chem. A. 2001, 105 (25)6144-6153.
- Useful monoprotected form of the dione. For use in a 3 step synthesis of 1-azaadamantan-4-one, see: Synthesis, 1080 (1992).