Search
Thermo Scientific Chemicals
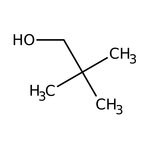
Neopentyl alcohol, 99%
CAS: 75-84-3 | C5H12O | 88.15 g/mol
Catalog number A14390.36
also known as A14390-36
Price (EUR)
328,95
Online Exclusive
387,00Save 58,05 (15%)
Each
Quantity:
500 g
Price (EUR)
328,95
Online Exclusive
387,00Save 58,05 (15%)
Each
Chemical Identifiers
CAS75-84-3
IUPAC Name2,2-dimethylpropan-1-ol
Molecular FormulaC5H12O
InChI KeyKPSSIOMAKSHJJG-UHFFFAOYSA-N
SMILESCC(C)(C)CO
View more
Specifications Specification Sheet
Specification Sheet
Identification (FTIR)Conforms
Assay (GC)≥98.5%
Appearance (Color)Colorless to white
Melting Point (clear melt)51.0-58.0°C
FormCrystals or powder or crystalline powder or lumps or chunks or fused solid
It is used in the Mitsunobu reaction of methyl salicylate coupling of sterically hindered substrates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used in the Mitsunobu reaction of methyl salicylate coupling of sterically hindered substrates.
Solubility
Soluble in water at (20°C 40 g/L), alcohol, and ether. Solubility in methanol is almost transparent.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It is used in the Mitsunobu reaction of methyl salicylate coupling of sterically hindered substrates.
Solubility
Soluble in water at (20°C 40 g/L), alcohol, and ether. Solubility in methanol is almost transparent.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Sudhakar Bhandaru, et al. Synthesis of a C14',15' dihydro derivative of the south hexacyclic steroid unit of cephalostatin 1. Part I : regiospecific Rh[II]-mediated intermolecular oxygen alkylation of a primary neopentyl alcohol.Tetrahedron Lett.,1995,36(46), 8347-8350.
- J. D. Slagle, et al. Mechanism of the triphenylphosphine-tetrachloromethane-alcohol reaction: pericyclic or clustered ion pairs?J. Org. Chem.,1981,46(17), 3526-3530.
- A method has been described for conversion to the iodide using a combination of methyl iodide and triphenyl phosphite: Org. Synth. Coll., 6, 830 (1988).