Search
Thermo Scientific Chemicals
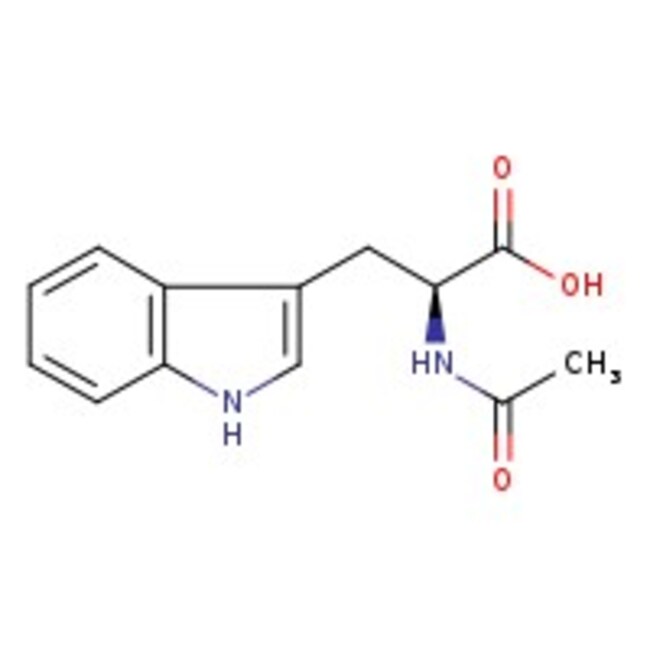
N-Acetyl-DL-tryptophan, 99%
CAS: 87-32-1 | C13H14N2O3 | 246.27 g/mol
Catalog number A17562.14
also known as A17562-14
Price (EUR)
32,30
Exklusiv online
38,00Save 5,70 (15%)
Each
Quantity:
25 g
Price (EUR)
32,30
Exklusiv online
38,00Save 5,70 (15%)
Each
Chemical Identifiers
CAS87-32-1
IUPAC Name2-acetamido-3-(1H-indol-3-yl)propanoic acid
Molecular FormulaC13H14N2O3
InChI KeyDZTHIGRZJZPRDV-UHFFFAOYSA-N
SMILESCC(=O)NC(CC1=CNC2=CC=CC=C12)C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream
FormCrystals or powder or crystalline powder
Assay (Aqueous acid-base Titration)≥98.0 to ≤102.0%
Assay (HPLC)≥98.0%
N-Acetyl-DL-tryptophan is produced via chemical synthesis using the standard amino acid, tryptophan. It can be used in downstream applications of biopharmaceutical production as a transfer agent or stabilizer.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Acetyl-DL-tryptophan is produced via chemical synthesis using the standard amino acid, tryptophan. It can be used in downstream applications of biopharmaceutical production as a transfer agent or stabilizer.
Solubility
Soluble in 1N Sodium Hydroxide, water, and methanol.
Notes
Keep container tightly closed. Store away from oxidizing agents.
N-Acetyl-DL-tryptophan is produced via chemical synthesis using the standard amino acid, tryptophan. It can be used in downstream applications of biopharmaceutical production as a transfer agent or stabilizer.
Solubility
Soluble in 1N Sodium Hydroxide, water, and methanol.
Notes
Keep container tightly closed. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- Harold Edelhoch. Spectroscopic Determination of Tryptophan and Tyrosine in Proteins. Biochemistry. 1967, 6, (7), 1948-1954.
- Ferenc J. Kezdy.; Gerald E. Clement.; Myron L. Bender. The Observation of Acyl-Enzyme Intermediates in the α-Chymotrypsin-Catalyzed Reactions of N-Acetyl-L-tryptophan Derivatives at Low pH. J. Am. Chem. Soc. 1964, 86, (18), 3690-3696.