Search
Thermo Scientific Chemicals
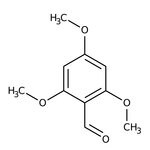
2,4,6-Trimethoxybenzaldehyde, 98%
CAS: 830-79-5 | C10H12O4 | 196.202 g/mol
Catalog number A11238.18
also known as A11238-18
Price (EUR)
165,75
Online Exclusive
195,00Save 29,25 (15%)
Each
Quantity:
50 g
Price (EUR)
165,75
Online Exclusive
195,00Save 29,25 (15%)
Each
Chemical Identifiers
CAS830-79-5
IUPAC Name2,4,6-trimethoxybenzaldehyde
Molecular FormulaC10H12O4
InChI KeyCRBZVDLXAIFERF-UHFFFAOYSA-N
SMILESCOC1=CC(OC)=C(C=O)C(OC)=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream
FormPowder or crystalline powder
Assay (GC)≥97.5%
Identification (FTIR)Conforms
Melting Point117-123°C
2,4,6-Trimethoxybenzaldehyde was used in the preparation and characterization of three RNA-specific fluorescent probes and their use in live cell imaging. It was used as starting reagent for the regioselective synthesis of new (±)-8-alkyl-5,7-dihydroxy-4-(4-hydroxyphenyl)-3,4-dihydrocoumarins.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,4,6-Trimethoxybenzaldehyde was used in the preparation and characterization of three RNA-specific fluorescent probes and their use in live cell imaging. It was used as starting reagent for the regioselective synthesis of new (+/-)-8-alkyl-5,7-dihydroxy-4-(4-hydroxyphenyl)-3,4-dihydrocoumarins.
Solubility
Insoluble in water.
Notes
Keep container tightly closed. Store away from oxidizing agents.
2,4,6-Trimethoxybenzaldehyde was used in the preparation and characterization of three RNA-specific fluorescent probes and their use in live cell imaging. It was used as starting reagent for the regioselective synthesis of new (+/-)-8-alkyl-5,7-dihydroxy-4-(4-hydroxyphenyl)-3,4-dihydrocoumarins.
Solubility
Insoluble in water.
Notes
Keep container tightly closed. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- Alexander McKillop, Duncan Kemp. Further functional group oxidations using sodium perborate. Tetrahedron. 1989, 45, (11), 3299-3306.
- Mark C. Munson.; Carlos Garcia-Echeverria.; Fernando Albericio.; George Barany. S-2,4,6-trimethoxybenzyl (Tmob): a novel cysteine protecting group for the N.alpha.-(9-fluorenylmethoxycarbonyl) (Fmoc) strategy of peptide synthesis. J. Org. Chem. 1992, 57, (11), 3013-3018.