Search
Thermo Scientific Chemicals
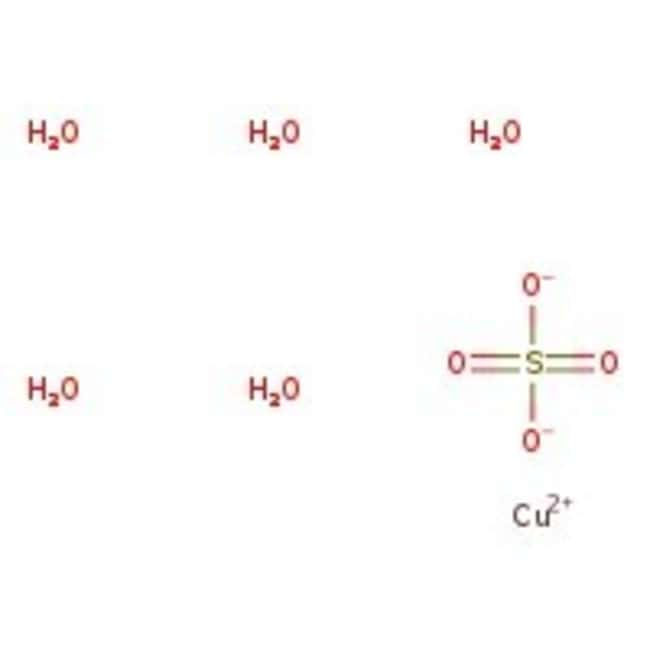
Copper(II) sulfate pentahydrate, 99%
CAS: 7758-99-8 | CuH10O9S | 249.68 g/mol
Catalog number A11262.0G
also known as A11262-0G
Price (EUR)
620,00
Each
Quantity:
25,000 g
Price (EUR)
620,00
Each
Chemical Identifiers
CAS7758-99-8
IUPAC Namecopper(2+) pentahydrate sulfate
Molecular FormulaCuH10O9S
InChI KeyJZCCFEFSEZPSOG-UHFFFAOYSA-L
SMILESO.O.O.O.O.[Cu++].[O-]S([O-])(=O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Blue
FormCrystals or crystalline powder and/or lumps
Assay (unspecified)>98.5 to <101.5%
Copper(II) sulfate, pentahydrate can be added with potassium permanganate to give an oxidant for the conversion of primary alcohols. It is used to etch zinc or copper plates for intaglio printmaking and as a mordant in vegetable dyeing. In analytical chemistry, it is used in Fehling's solution and Benedict's solution to test for reducing sugars. Also, used to test protein in Biuret test.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Copper(II) sulfate, pentahydrate can be added with potassium permanganate to give an oxidant for the conversion of primary alcohols. It is used to etch zinc or copper plates for intaglio printmaking and as a mordant in vegetable dyeing. In analytical chemistry, it is used in Fehling′s solution and Benedict′s solution to test for reducing sugars. Also, used to test protein in Biuret test.
Solubility
Soluble in water and methanol. Insoluble in alcohol.
Notes
Incompatible with steel, finely powdered metals, hydroxylamine, magnesium, hydrazine, nitromethane, alkalies, phosphates and acetylene gas
Copper(II) sulfate, pentahydrate can be added with potassium permanganate to give an oxidant for the conversion of primary alcohols. It is used to etch zinc or copper plates for intaglio printmaking and as a mordant in vegetable dyeing. In analytical chemistry, it is used in Fehling′s solution and Benedict′s solution to test for reducing sugars. Also, used to test protein in Biuret test.
Solubility
Soluble in water and methanol. Insoluble in alcohol.
Notes
Incompatible with steel, finely powdered metals, hydroxylamine, magnesium, hydrazine, nitromethane, alkalies, phosphates and acetylene gas
RUO – Research Use Only
General References:
- Ford, D. D.; Lenahan, S.; Jorgensen, M.; Dube, P.; Delude, M.; Concannon, P. E.; Anderson, S. R.; Oyler, K. D.; Cheng, G.; Mehta, N.; Salan, J. S. Development of a Lean Process to the Lead-Free Primary Explosive DBX-1. Org. Process Res. Dev. 2015, 19 (6), 673-680.
- Golf, H. R. A.; Reissig, H.; Wiehe, A. Synthesis of SF5-Substituted Tetrapyrroles, Metalloporphyrins, BODIPYs, and Their Dipyrrane Precursors. J. Org. Chem. 2015, 80 (10), 5133-5143.

.png-150.jpg)



