Search
Thermo Scientific Chemicals
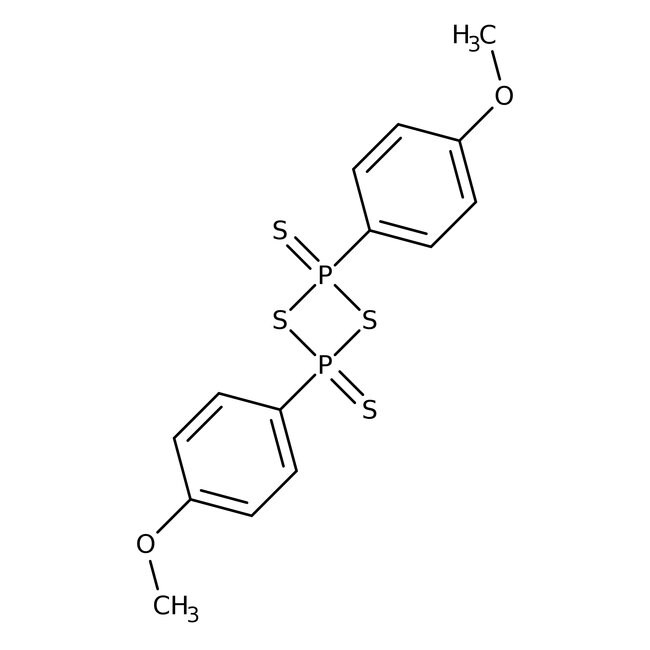
Lawesson's Reagent, 97%
CAS: 19172-47-5 | C14H14O2P2S4 | 404.452 g/mol
Catalog number A14530.22
also known as A14530-22
Price (EUR)
113,05
Online Exclusive
133,00Save 19,95 (15%)
Each
Quantity:
100 g
Price (EUR)
113,05
Online Exclusive
133,00Save 19,95 (15%)
Each
Chemical Identifiers
CAS19172-47-5
IUPAC Namebis(4-methoxyphenyl)-1,3,2λ⁵,4λ⁵-dithiadiphosphetane-2,4-dithione
Molecular FormulaC14H14O2P2S4
InChI KeyCFHGBZLNZZVTAY-UHFFFAOYSA-N
SMILESCOC1=CC=C(C=C1)P1(=S)SP(=S)(S1)C1=CC=C(OC)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream or pale yellow
FormCrystals or powder or crystalline powder
Melting Point218-226?C
Assay (Aqueous acid-base Titration)≥96.0 to ≤104.0%
Lawesson's reagent is a thiation agent used to convert carbonyl compounds into thiocarbonyls. It is also used to thionate enones, esters, lactones, amides, lactams and quinones. Further, it is used to prepare thiols from alcohols. It is associated with silver perchlorate and utilized as and oxophilic Lewis acid catalyst for Diels-Alder reaction of dienes with alfa, beta-unsaturated aldehydes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Lawesson′s reagent is a thiation agent used to convert carbonyl compounds into thiocarbonyls. It is also used to thionate enones, esters, lactones, amides, lactams and quinones. Further, it is used to prepare thiols from alcohols. It is associated with silver perchlorate and utilized as and oxophilic Lewis acid catalyst for Diels-Alder reaction of dienes with alfa, beta-unsaturated aldehydes.
Solubility
Insoluble in water.
Notes
Incompatible with strong oxidizing agents.
Lawesson′s reagent is a thiation agent used to convert carbonyl compounds into thiocarbonyls. It is also used to thionate enones, esters, lactones, amides, lactams and quinones. Further, it is used to prepare thiols from alcohols. It is associated with silver perchlorate and utilized as and oxophilic Lewis acid catalyst for Diels-Alder reaction of dienes with alfa, beta-unsaturated aldehydes.
Solubility
Insoluble in water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Powerful thionation agent used for the replacement of the carbonyl oxygen of ketones, amides and esters, by sulfur: Bull. Soc. Chim. Belg., 87, 223, 229, 293, 299, 525 (1978); Tetrahedron, 35, 1339, 2433 (1979). Review: Tetrahedron, 41, 5061 (1985). For conversion of N-methylpyrrolidinone to the thiolactam, see: Org. Synth. Coll., 7, 372 (1990).
- Uracil and its derivatives are thionated selectively at the 4-position: Synthesis, 152 (1988). Peptides give endo-thiopeptides: Tetrahedron, 37, 3635 (1981). Esters, orthoesters, acetals and epoxides give the thio-analogues: Monatsh. Chem., 115, 769 (1984). Used, in combination with 1,1,3,3-Tetramethyl thiourea, L13392, for formation of bis-thioesters for subsequent radical coupling as a method of ring formation in Nicolaou's syntheses of hemibrevitoxin B and brevitoxin B: J. Am. Chem. Soc., 115, 3558 (1993); 117, 10227 (1995).
- Reaction with 1,4-diketones gives 2,5-disubstituted thiophenes in high yield: Synthesis, 1061 (1982).
- Reaction with ɑ-diazo ketones has been used to prepare 1,2,3-thiadiazoles: Heterocycles, 19, 241 (1982).
- Benzylic and related alcohols are converted to the corresponding thiols: J. Chem. Soc., Chem. Commun., 205 (1989); J. Chem. Soc., Perkin 1, 1113 (1993).
- Reacts with N-protected amino acids to give mixed anhydrides, useful in peptide coupling: Tetrahedron, 38, 3267 (1982). See Appendix 6.
- Has also been found to act as an efficient trapping agent for 1,3-dipoles under very mild conditions. Its powerful dipolarophile character allows it to be used as a 1,3-dipole indicator even with dipoles of low reactivity, the outcome of the reaction being readily verified by 31P NMR: J. Org. Chem., 60, 3904 (1995).