Search
Thermo Scientific Chemicals
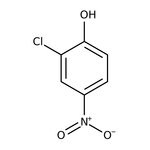
2-Chloro-4-nitrophenol, 97%
CAS: 619-08-9 | C6H4ClNO3 | 173.552 g/mol
Catalog number B21561.30
also known as B21561-30
Price (EUR)
331,00
Each
Quantity:
250 g
Price (EUR)
331,00
Each
Chemical Identifiers
CAS619-08-9
IUPAC Name2-chloro-4-nitrophenol
Molecular FormulaC6H4ClNO3
InChI KeyBOFRXDMCQRTGII-UHFFFAOYSA-N
SMILESOC1=CC=C(C=C1Cl)[N+]([O-])=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream to pale yellow to pale brown to gray
Assay (GC)≥96.0%
Melting Point (clear melt)107.0-116.0?C
FormPowder
2-Chloro-4-nitrophenol, is frequently used as building blocks for dyes, plastics, explosives. It acts as a catalytic agent, petrochemical additive and used in organic synthesis. It can react with sulfuric acid dimethyl ester to produce 2-chloro-4-nitro-anisole. This reaction will need reagents K2CO3 and xylene.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Chloro-4-nitrophenol, is frequently used as building blocks for dyes, plastics, explosives. It acts as a catalytic agent, petrochemical additive and used in organic synthesis. It can react with sulfuric acid dimethyl ester to produce 2-chloro-4-nitro-anisole. This reaction will need reagents K2CO3 and xylene.
Solubility
Slightly Soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away strong oxidizing agents, strong bases.
2-Chloro-4-nitrophenol, is frequently used as building blocks for dyes, plastics, explosives. It acts as a catalytic agent, petrochemical additive and used in organic synthesis. It can react with sulfuric acid dimethyl ester to produce 2-chloro-4-nitro-anisole. This reaction will need reagents K2CO3 and xylene.
Solubility
Slightly Soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away strong oxidizing agents, strong bases.
RUO – Research Use Only
General References:
- S Teshima.; N Mitsuhida.; M Ando. Determination of α-amylase in biological fluids using a new substrate (β-2-chloro-4-nitrophenyl-maltopentaoside). Clinica chimica acta. 1985150 (3), 165-174.
- PK Arora.; RK Jain. Pathway for degradation of 2-chloro-4-nitrophenol in Arthrobacter sp. SJCon. Current microbiology. 20115 (3), 405-411.