Search Thermo Fisher Scientific
2-Bromoisobutyryl bromide, 97%, Thermo Scientific Chemicals
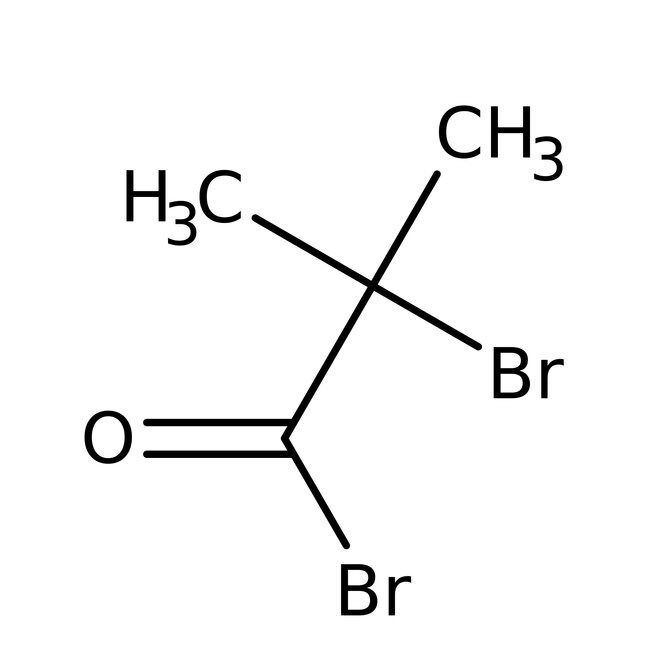

2-Bromoisobutyryl bromide, 97%, Thermo Scientific Chemicals
Chemical Identifiers
Specifications
Description
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
2-Bromoisobutyryl bromide is used to prepare N-protected halodienamide, which provides four- and five-membered lactams in the presence of copper(I) and a tertiary amine. Further, it is used as atom transfer radical polymerization(ATRP) initiator for functionalization of hydroxyl groups present on the surface of graphene oxide. It is also used in preparation of polycaprolactone macroinitiators through reaction with oligomeric caprolactone diol and mesoporous silica nanoparticles with ATRP initiator anchored on the exterior surface.
Solubility
Miscible with acetone and carbon disulfide.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents and strong alkalis.
Figures
Documents & Downloads
Certificates
Frequently asked questions (FAQs)
Citations & References
Safety and Handling
Classification of the substance or mixture
CLP classification - Regulation(EC) No 1272/2008
Label Elements
Signal Word
Danger
Hazard Statements
H302 - Harmful if swallowed
H314 - Causes severe skin burns and eye damage
H317 - May cause an allergic skin reaction
H340 - May cause genetic defects
Precautionary Statements
P280 - Wear protective gloves/protective clothing/eye protection/face protection
P301 + P330 + P331 - IF SWALLOWED: Rinse mouth. Do NOT induce vomiting
P303 + P361 + P353 - IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water or shower
P305 + P351 + P338 - IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
P310 - Immediately call a POISON CENTER or doctor/physician
Additional EU labelling
Restricted to professional users