Search
Thermo Scientific Chemicals
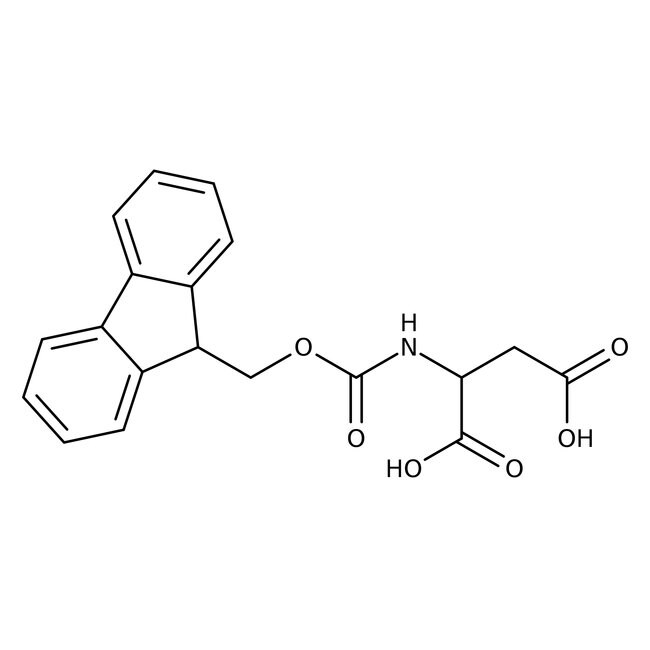
N-Fmoc-L-aspartic acid, 95%
CAS: 119062-05-4 | C19H17NO6 | 355.346 g/mol
Catalog number H62274.06
also known as H62274-06
Price (EUR)
50,30
Each
Quantity:
5 g
Price (EUR)
50,30
Each
Chemical Identifiers
CAS119062-05-4
IUPAC Name2-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)butanedioic acid
Molecular FormulaC19H17NO6
InChI KeyKSDTXRUIZMTBNV-UHFFFAOYNA-N
SMILESOC(=O)CC(NC(=O)OCC1C2=CC=CC=C2C2=CC=CC=C12)C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Assay (HPLC)>94.0%
N-Fmoc-L-aspartic acid is used to biosynthesize other amino acids with in the human body.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Fmoc-L-aspartic acid is used to biosynthesize other amino acids with in the human body.
Solubility
Soluble in methanol, and water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
N-Fmoc-L-aspartic acid is used to biosynthesize other amino acids with in the human body.
Solubility
Soluble in methanol, and water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- Paul A. Keifer. Influence of Resin Structure, Tether Length, and Solvent upon the High-Resolution 1H NMR Spectra of Solid-Phase-Synthesis Resins. J. Org. Chem. 1996, 61, (5), 1558-1559.
- W. John Cooper.; Marcey L. Waters. Turn Residues in β-Hairpin Peptides as Points for Covalent Modification. Org. Lett. 2005, 7, (18), 3825-3828.