Search
Thermo Scientific Chemicals
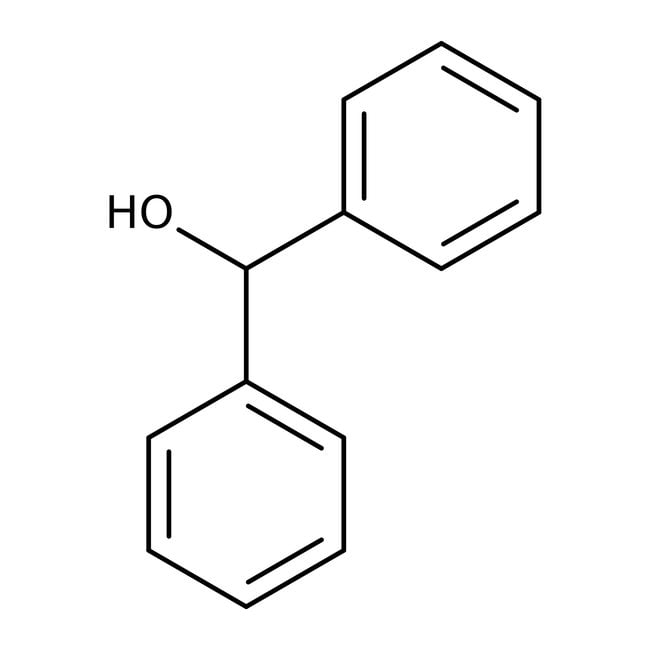
Bencidrol, 99 %, Thermo Scientific Chemicals
CAS: 91-01-0 | C13H12O | 184.238 g/mol
Número de catálogo A12884.0B
también denominado A12884-0B
Precio (EUR)
113,90
Online Exclusive
134,00Ahorro 20,10 (15%)
Each
Cantidad:
1000 g
Precio (EUR)
113,90
Online Exclusive
134,00Ahorro 20,10 (15%)
Each
Identificadores químicos
CAS91-01-0
IUPAC Namediphenylmethanol
Molecular FormulaC13H12O
InChI KeyQILSFLSDHQAZET-UHFFFAOYSA-N
SMILESOC(C1=CC=CC=C1)C1=CC=CC=C1
Ver más
Especificaciones Hoja de especificaciones
Hoja de especificaciones
Appearance (Color)White
Assay (GC)≥98.5%
Melting Point (clear melt)63.0-70.0?C
FormCrystals or powder or crystalline powder
Identification (FTIR)Conforms
Benzhydrol is widely used as intermediates in pharmaceuticals (including antihistamines), agrochemicals, perfumes and other organic compounds. It is used as a fixative in the perfume industry. It is involved in polymerization reaction as a terminating group. It is used as precursor to prepare modafinil, benztropine and diphehydramine.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Aplicaciones
El bencidrol se utiliza ampliamente como intermediario en productos farmacéuticos (entre otros, los antihistamínicos), agroquímicos, perfumes y otros compuestos orgánicos. Se utiliza como fijador en la industria del perfume. Participa en la reacción de polimerización como grupo terminal. Se utiliza como precursor para preparar modafinilo, benzatropina y difenhidramina.
Solubilidad
Ligeramente soluble en agua.
Notas
Incompatible con agentes oxidantes fuertes, ácidos, cloruros y anhídridos ácidos.
El bencidrol se utiliza ampliamente como intermediario en productos farmacéuticos (entre otros, los antihistamínicos), agroquímicos, perfumes y otros compuestos orgánicos. Se utiliza como fijador en la industria del perfume. Participa en la reacción de polimerización como grupo terminal. Se utiliza como precursor para preparar modafinilo, benzatropina y difenhidramina.
Solubilidad
Ligeramente soluble en agua.
Notas
Incompatible con agentes oxidantes fuertes, ácidos, cloruros y anhídridos ácidos.
RUO – Research Use Only
General References:
- The protection of OH groups as benzhydryl ethers can be catalyzed by sulfuric acid: Org. Synth. Coll., 4, 72 (1963); Angew. Chem. Int. Ed., 15, 281 (1976). See also Benzhydryl bromide, L02211. Thiols, e.g. in cysteine residues in peptide synthesis, can be protected as thioethers in the presence of TFA or HBr/AcOH: J. Chem. Soc. (C), 2683 (1970). The group can be cleaved by TFA in the presence of a cation scavenger, e.g. phenol or anisole (same ref.), or by Na in liquid NH3: J. Am. Chem. Soc., 84, 3887 (1962). See Appendix 6.
- Carboxyl groups can be protected by esterification in benzene with tosic acid catalyst. Deprotection can be effected with the same catalyst by refluxing in toluene, which undergoes electrophilic alkylation: Tetrahedron Lett., 37, 1965 (1996).
- Misawa, T.; Dodo, K.; Ishikawa, M.; Hashimoto, Y.; Sagawa, M.; Kizaki, M.; Aoyama, H. Structure-activity relationships of benzhydrol derivatives based on 1'-acetoxychavicol acetate (ACA) and their inhibitory activities on multiple myeloma cell growth via inactivation of the NF-κB pathway. Bioorg. Med. Chem. 2015, 23 (9), 2241-2246.
- Taskin, O. S.; Temel, B. A.; Tasdelen, M. A.; Yagci, Y. Synthesis of block copolymers by selective H-abstraction and radical coupling reactions using benzophenone/benzhydrol photoinitiating system. Eur. Polym. J. 2015, 62, 304-311.