Search
Thermo Scientific Chemicals
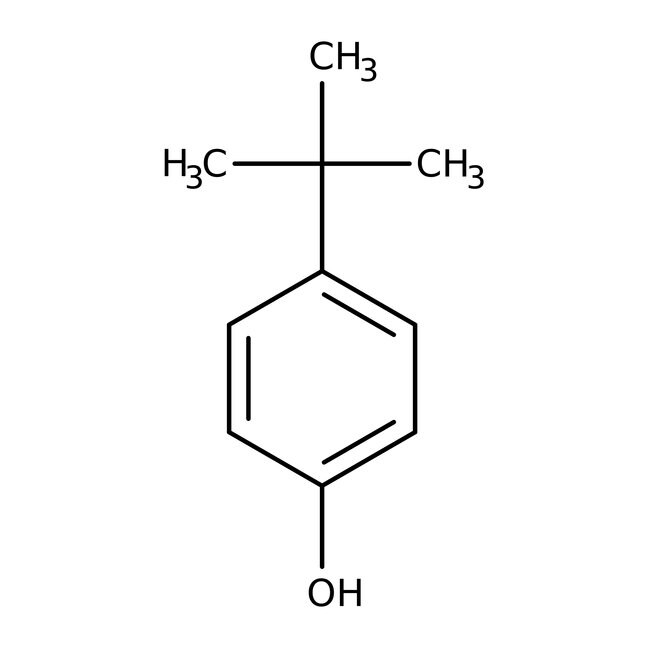
4-terc-Butilfenol, 99 %, Thermo Scientific Chemicals
CAS: 98-54-4 | C10H14O | 150.221 g/mol
Número de catálogo A15871.0E
también denominado A15871-0E
Precio (EUR)
83,80
Each
Cantidad:
2500 g
Precio (EUR)
83,80
Each
Identificadores químicos
CAS98-54-4
IUPAC Name4-tert-butylphenol
Molecular FormulaC10H14O
InChI KeyQHPQWRBYOIRBIT-UHFFFAOYSA-N
SMILESCC(C)(C)C1=CC=C(O)C=C1
Ver más
Especificaciones Hoja de especificaciones
Hoja de especificaciones
Appearance (Color)White to pale cream or pale yellow
FormPowder, flakes, briquettes or lumps
Melting Point (clear melt)97-101?C
Assay (GC)≥98.5%
Identification (FTIR)Conforms
4-tert-butylphenol on condensation with formaldehyde gives calix[5]arene which is used in enzyme mimetics.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Aplicaciones
El 4-terc-butilfenol en condensación con el formaldehído da calix[5]areno que se utiliza en la mimética enzimática.
Solubilidad
Soluble en agua. [8,7 g/l (20 °C)]
Notas
Almacenar a temperatura ambiente. Manténgase lejos de agentes oxidantes y ácidos.
El 4-terc-butilfenol en condensación con el formaldehído da calix[5]areno que se utiliza en la mimética enzimática.
Solubilidad
Soluble en agua. [8,7 g/l (20 °C)]
Notas
Almacenar a temperatura ambiente. Manténgase lejos de agentes oxidantes y ácidos.
RUO - Para uso exclusivo en investigación
- C. David Gutsche; Balram Dhawan; Kwang Hyun No; Ramamurthi Muthukrishnan. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J. Am. Chem. Soc. 1981, 103 (13),3782-3792 .
- The acid-catalyzed removal of t-butyl groups from phenols (reverse Friedel-Crafts reaction), can be brought about without the need for t-butyl acceptors by using aluminum chloride as catalyst in dichloromethane at ambient temperature: Synth. Commun., 18, 1783 (1988).