Search
Thermo Scientific Chemicals
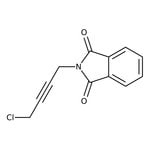
N-(4-Chloro-2-butynyl)phthalimide, 97%
CAS: 4819-69-6 | C12H8ClNO2 | 233.651 g/mol
Catalog number L16313.03
also known as L16313-03
Price (EUR)
87,70
Each
Quantity:
1 g
Price (EUR)
87,70
Each
Chemical Identifiers
CAS4819-69-6
IUPAC Name2-(4-chlorobut-2-yn-1-yl)-2,3-dihydro-1H-isoindole-1,3-dione
Molecular FormulaC12H8ClNO2
InChI KeyTXNDRPKNOXQAAO-UHFFFAOYSA-N
SMILESClCC#CCN1C(=O)C2=CC=CC=C2C1=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream
FormCrystals or powder or crystalline powder
Melting Point (clear melt)115.0-121.0?C
Assay (HPLC)≥96.0%
N-(4-Chloro-2-butynyl)phthalimide is used to make other chemicals and as pharmaceutical intermediate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-(4-Chloro-2-butynyl)phthalimide is used to make other chemicals and as pharmaceutical intermediate.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong bases and strong oxidizing agents.
N-(4-Chloro-2-butynyl)phthalimide is used to make other chemicals and as pharmaceutical intermediate.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong bases and strong oxidizing agents.
RUO – Research Use Only
General References:
- John L. Neumeyer, Urve V. Moyer, Janice A. Richman, Frank J. Rosenberg, David G. Teiger. Pharmacologically Active Acetylene Compounds. I.1,2 Structural Modifications of Oxotremorine. J. Med. Chem. 1967, 10 (4), 615-620.
- Alvie L. Davis, Rodney Lloyd, Stephen Maul, Drusilla E. Cook, Tommy J. McCord. 2,6-Diamino-4-hexynoic acid, a lysine analog. Archives of Biochemistry and Biophysics. 1964, 104 (2), 238-240.