Search
인용 및 참조 문헌 (5)
Thermo Scientific™
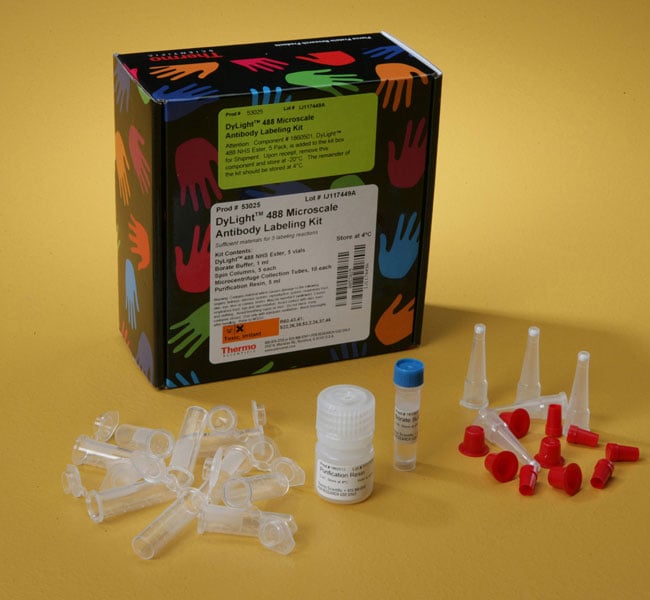
DyLight™ Microscale Antibody Labeling Kits
Label small batches of antibody for fluorescence microscopy, flow cytometry, IHC, western blots, ELISA, and more with DyLight Antibody Labeling kits.
| 카탈로그 번호 | 여기/방출 | 라벨 또는 염료 |
|---|---|---|
| 84536 | 652/672 | DyLight 650 |
| 53021 | 401/421 | DyLight 405 |
| 53025 | 490/525 | DyLight 488 |
| 53045 | 590/617 | DyLight 594 |
| 53063 | 784/814 | DyLight 800 |
| 84531 | 562/576 | DyLight 550 |
| 84539 | 749/775 | DyLight 755 |
카탈로그 번호 84536
제품 가격(KRW)
683,000
Online offer
Ends: 31-Mar-2026
803,000할인액 120,000 (15%)
Each
여기/방출:
652/672
라벨 또는 염료:
DyLight 650
제품 가격(KRW)
683,000
Online offer
Ends: 31-Mar-2026
803,000할인액 120,000 (15%)
Each
Invitrogen Dylight Microscale Antibody Labeling kits provide everything needed to label 100 μg of antibody or protein with amine-reactive Dylight dye and subsequent free dye removal. Choose from a variety of Dylight fluorophores across the spectrum to covalently label your antibody or protein. Labeled antibodies are typically ready to use in 90 minutes with minimal hands-on time. The kit contains all necessary components to perform five separate labeling reactions using 100 μg of IgG or similar quantities of other proteins. Labeled antibody or protein can be used for a variety of applications including immunofluorescence microscopy, flow cytometry, IHC, western blotting, or ELISA.
Invitrogen DyLight Antibody Labeling kits enable the creation of fluorescent antibodies that are covalently linked to different DyLight fluorophores. The fluorescently labeled antibodies can be used in a wide variety of laboratory applications, including immunofluorescence microscopy, IHC, western blotting, ELISA, flow cytometry, or in vitro and in vivo fluorescent detection strategies.
DyLight Antibody Labeling kits contain an NHS ester-activated derivative of the high-performance DyLight dye that can be used to fluorescently label antibodies and other proteins for cellular imaging and other fluorescence detection methods. The DyLight dye is an amine-reactive dye and is activated with an N-hydroxysuccinimide (NHS) ester moiety to react with exposed N-terminal α-amino groups or the ε-amino groups of lysine residues to form stable amide bonds. DyLight Antibody Labeling kits contain all necessary components to perform three separate labeling reactions and free dye removal using 100 μg of IgG or similar quantities of other proteins.
DyLight dyes exhibit higher fluorescence intensity and photostability than CyDye and LI-COR™ dyes in many applications and remain highly fluorescent over a broad pH range (pH 4–9). Additionally, the water solubility of the DyLight fluorescent dyes allows a high dye-to-protein ratio to be achieved without causing precipitation of conjugates. Finally, the high solubility of these fluorophores permits protein solutions to be added directly to specific amounts of the labeling reagent.
Other advantages of DyLight Antibody Labeling kits:
· High performance—high fluorescence intensity across a broad pH range
· Multiple colors—10 different dyes across the visible spectrum and extending into the infrared
· Specificity—NHS ester-activated dye labels proteins and other molecules at primary amines (-NH2)
· Optimized procedure—following the standard protocol results in antibodies with excellent dye:protein ratios and recovery rates for optimum activity and fluorescence labeling
We also offer DyLight Antibody Labeling kits for fast and efficient fluorescent labeling of antibodies for use in fluorescence methods. DyLight Antibody Labeling kits contain all necessary components to perform three separate labeling reactions using 1 mg of IgG or similar quantities of other proteins. Both the standard and microscale kit sizes include the amine-reactive DyLight fluorophore in convenient single-use vials as well as purification resin and spin columns for the preparation of ready-to-use conjugate.
DyLight Antibody Labeling kits contain an NHS ester-activated derivative of the high-performance DyLight dye that can be used to fluorescently label antibodies and other proteins for cellular imaging and other fluorescence detection methods. The DyLight dye is an amine-reactive dye and is activated with an N-hydroxysuccinimide (NHS) ester moiety to react with exposed N-terminal α-amino groups or the ε-amino groups of lysine residues to form stable amide bonds. DyLight Antibody Labeling kits contain all necessary components to perform three separate labeling reactions and free dye removal using 100 μg of IgG or similar quantities of other proteins.
DyLight dyes exhibit higher fluorescence intensity and photostability than CyDye and LI-COR™ dyes in many applications and remain highly fluorescent over a broad pH range (pH 4–9). Additionally, the water solubility of the DyLight fluorescent dyes allows a high dye-to-protein ratio to be achieved without causing precipitation of conjugates. Finally, the high solubility of these fluorophores permits protein solutions to be added directly to specific amounts of the labeling reagent.
Other advantages of DyLight Antibody Labeling kits:
· High performance—high fluorescence intensity across a broad pH range
· Multiple colors—10 different dyes across the visible spectrum and extending into the infrared
· Specificity—NHS ester-activated dye labels proteins and other molecules at primary amines (-NH2)
· Optimized procedure—following the standard protocol results in antibodies with excellent dye:protein ratios and recovery rates for optimum activity and fluorescence labeling
We also offer DyLight Antibody Labeling kits for fast and efficient fluorescent labeling of antibodies for use in fluorescence methods. DyLight Antibody Labeling kits contain all necessary components to perform three separate labeling reactions using 1 mg of IgG or similar quantities of other proteins. Both the standard and microscale kit sizes include the amine-reactive DyLight fluorophore in convenient single-use vials as well as purification resin and spin columns for the preparation of ready-to-use conjugate.
For Research Use Only. Not for use in diagnostic procedures.
사양
색상Far-Red
여기/방출652/672
키트 구성품Contains DyLight Fluor NHS Ester, 5 x 15 μg vials; Borate Buffer (0.67 M), 1 mL; Purification Resin, 5 mL; Microcentrifuge Spin Columns, 5 columns; and Microcentrifuge Collection Tubes, 10 Tubes
라벨 유형DyLight™
라벨링 스케일100 μg
제품라인DyLight
제품 유형Labeling Kit
수량5 reactions
화학물질 반응성Amine
Labeling TargetAntibodies, Proteins
라벨 또는 염료DyLight 650
Unit SizeEach
구성 및 보관
Store the DyLight NHS Ester at -20°C. Store all other kit components at 4°C.
인용 및 참조 문헌 (5)
인용 및 참조 문헌
Abstract
Unraveling mitotic protein networks by 3D multiplexed epitope drug screening.
Journal:Mol Syst Biol
PubMed ID:30104419
'Three-dimensional protein localization intricately determines the functional coordination of cellular processes. The complex spatial context of protein landscape has been assessed by multiplexed immunofluorescent staining or mass spectrometry, applied to 2D cell culture with limited physiological relevance or tissue sections. Here, we present 3D SPECS, an automated technology for 3D
Inducible IFN-? Expression for MHC-I Upregulation in Devil Facial Tumor Cells.
Journal:Front Immunol
PubMed ID:30692995
'The Tasmanian devil facial tumor (DFT) disease has led to an 80% reduction in the wild Tasmanian devil ('
Development by Genetic Immunization of Monovalent Antibodies Against Human Vasoactive Intestinal Peptide Receptor 1 (VPAC1), New Innovative, and Versatile Tools to Study VPAC1 Receptor Function.
Journal:Front Endocrinol (Lausanne)
PubMed ID:29674997
Multi-membrane spanning proteins, such as G protein-coupled receptors (GPCRs) and ion channels, are extremely difficult to purify as native proteins. Consequently, the generation of antibodies that recognize the native conformation can be challenging. By combining genetic immunization, phage display, and biopanning, we identified a panel of monovalent antibodies (nanobodies) targeting
Cell-free protein synthesis as a novel tool for directed glycoengineering of active erythropoietin.
Journal:Sci Rep
PubMed ID:29867209
As one of the most complex post-translational modification, glycosylation is widely involved in cell adhesion, cell proliferation and immune response. Nevertheless glycoproteins with an identical polypeptide backbone mostly differ in their glycosylation patterns. Due to this heterogeneity, the mapping of different glycosylation patterns to their associated function is nearly impossible.
High-Affinity Bent ß
Journal:Cell Rep
PubMed ID:30605669
Leukocyte adhesion requires ß