Search
인용 및 참조 문헌 (3)
Gibco™
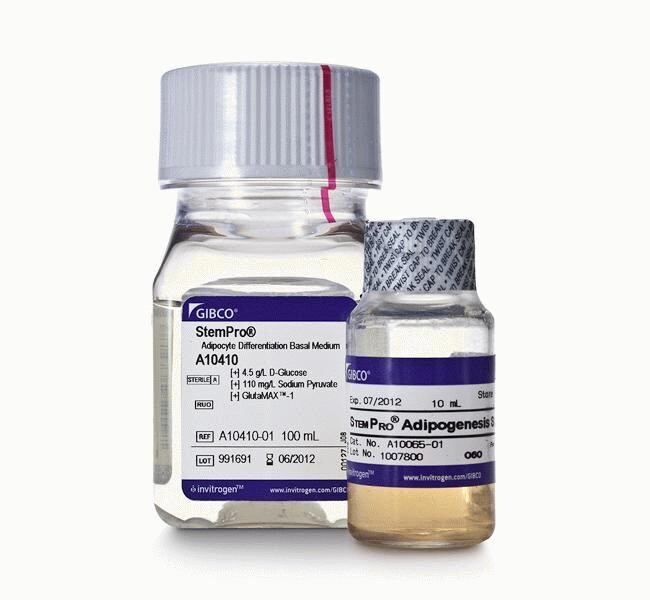
StemPro™ Adipogenesis Differentiation Kit
인간 중간엽 줄기세포를 지방세포로 완전히 분화시킴StemPro™ Adipogenesis Differentiation Kit는 조직 배양 용기에서 중간엽줄기세포(MSC)의 지방세포 분화를 위해 개발되었습니다. 이 kit에는 MSC가자세히 알아보기
| 카탈로그 번호 | 수량 |
|---|---|
| A1007001 | 1 Kit |
카탈로그 번호 A1007001
제품 가격(KRW)
285,000
Online offer
Ends: 31-Mar-2026
316,000할인액 31,000 (10%)
Each
수량:
1 Kit
제품 가격(KRW)
285,000
Online offer
Ends: 31-Mar-2026
316,000할인액 31,000 (10%)
Each
인간 중간엽 줄기세포를 지방세포로 완전히 분화시킴
StemPro™ Adipogenesis Differentiation Kit는 조직 배양 용기에서 중간엽줄기세포(MSC)의 지방세포 분화를 위해 개발되었습니다. 이 kit에는 MSC가 지방생성 경로에 기여하여 지방세포를 생성하도록 하는데 필요한 모든 시약이 들어있습니다. StemPro™ Adipogenesis Differentiation Kit와 StemPro™ MSC SFM 또는 MesenPRO RS™ Medium를 함께 사용하여 MSC 분리, 확장, 지질 소포체 형성 지방세포 분화에 필요한 표준 배양 작업 용액을 만들 수 있습니다.
StemPro™ Adipogenesis Differentiation Kit는 조직 배양 용기에서 중간엽줄기세포(MSC)의 지방세포 분화를 위해 개발되었습니다. 이 kit에는 MSC가 지방생성 경로에 기여하여 지방세포를 생성하도록 하는데 필요한 모든 시약이 들어있습니다. StemPro™ Adipogenesis Differentiation Kit와 StemPro™ MSC SFM 또는 MesenPRO RS™ Medium를 함께 사용하여 MSC 분리, 확장, 지질 소포체 형성 지방세포 분화에 필요한 표준 배양 작업 용액을 만들 수 있습니다.
For Research Use Only. Not for use in diagnostic procedures.
사양
세포 유형Stem Cells (Mesenchymal)
배양 환경CO2
엔도톡신 수준≤50 EU/mL, ≤ 50 EU/ml
형식Liquid
글루코스High Glucose
글루타민GlutaMAX™-I
HEPES 버퍼No HEPES
무기 염류Calcium, Sodium Phosphate
페놀 레드 인디케이터No Phenol Red
순도 또는 품질 등급Research Grade
수량1 Kit
연구 카테고리Differentiation
혈청 수준Standard Serum
Sodium Bicarbonate 버퍼Sodium Bicarbonate
Sodium Pyruvate 첨가제Sodium Pyruvate
종Human
보충제Adipogenesis Supplements
용도(애플리케이션)Stem Cell Differentiation
제품라인Gibco, StemPro
제품 유형Adipogenesis Differentiation Kit
Unit SizeEach
구성 및 보관
This kit is shipped in two parts with separate storage requirements. These components are not sold individually:
• StemPro™ Adipocyte Differentiation Basal Medium (100 ml; store in the dark at 2°C to 8°C)
• StemPro™ Adipogenesis Supplement (10 ml; store in the dark at -5°C to -20°C)
The Basal Medium ships at room temperature, while the Supplement ships on dry ice.
• StemPro™ Adipocyte Differentiation Basal Medium (100 ml; store in the dark at 2°C to 8°C)
• StemPro™ Adipogenesis Supplement (10 ml; store in the dark at -5°C to -20°C)
The Basal Medium ships at room temperature, while the Supplement ships on dry ice.
자주 묻는 질문(FAQ)
What kinds of growth factors are used in mesenchymal stem cell (MSC) research?
How can I culture mesenchymal stem cells (MSCs) in vitro?
How do you characterize human mesenchymal stem cells (MSCs)?
What are mesenchymal stem cells?
인용 및 참조 문헌 (3)
인용 및 참조 문헌
Abstract
Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media.
Journal:
PubMed ID:26564250
'Due to their immunosuppressive properties, mesenchymal stem cells (MSC) have been evaluated for the treatment of immunological diseases. However, the animal-derived growth supplements utilized for MSC manufacturing may lead to clinical complications. Characterization of alternative media formulations is imperative for MSC therapeutic application. Human BMMSC and AdMSC were expanded in
Alginate-Encapsulation for the Improved Hypothermic Preservation of Human Adipose-Derived Stem Cells.
Journal:Stem Cells Transl Med
PubMed ID:26826163
: Despite considerable progress within the cell therapy industry, unmet bioprocessing and logistical challenges associated with the storage and distribution of cells between sites of manufacture and the clinic exist. We examined whether hypothermic (4°C-23°C) preservation of human adipose-derived stem cells could be improved through their encapsulation in 1.2% calcium
Human Synovial MSC Good Manufacturing Practices for Articular Cartilage Regeneration.
Journal:Tissue Eng Part C Methods
PubMed ID:30412046
cartilage restoration is a desperately needed bridge for patients with symptomatic cartilage lesions. Chondral lesion is a pathology with high prevalence, reaching as much as 63% of general population and 36% among athletes. Despite Autologous Chondrocyte Implantation (ACI) versatility, it still fails to fully reproduce hyaline articular cartilage characteristics. Mesenchymal