Search
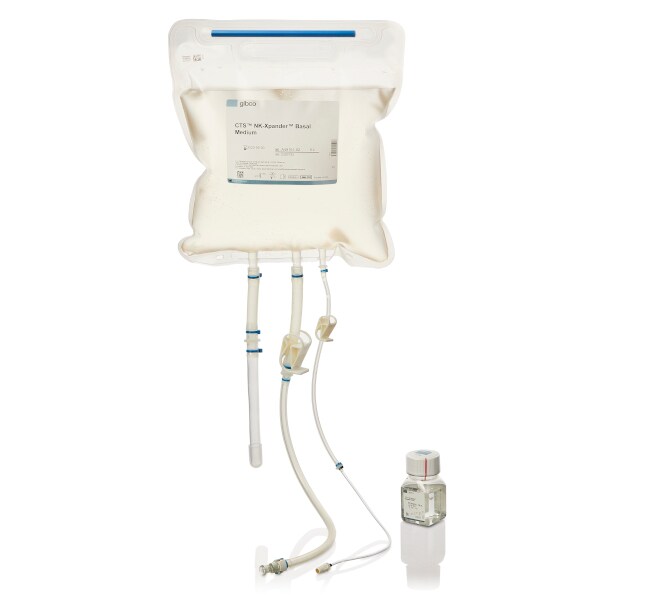
CTS™ NK-Xpander™ Medium
| Catalog Number | Quantity | Format |
|---|---|---|
| A5019002 | 5 L | BPC |
| A5019001 | 500 mL | Bottle |
| A5019005 | 5 L | BPC, Bagged Supplement (100 mL) |
| A5019003 | 10 L | BPC |
| A5019004 | 20 L | BPC |
Gibco CTS NK-Xpander Medium is specifically formulated for superior expansion of human natural killer (hNK) cells for cell therapy applications. When hNK cells are enriched from human peripheral blood mononuclear cells (hPBMCs) and expanded in a feeder-free system using CTS NK-Xpander Medium supplemented with 5% hAB serum and 500U/mL IL-2, ≥90% of the hNK cells generated maintain CD56+/CD16+/CD3- surface marker expression. Furthermore, such hNK cells demonstrate degranulation and killing of K562 cancer cells when expanded in CTS NK-Xpander Medium.
Superior expansion potential
CTS NK-Xpander Medium is capable of producing a high yield of hNK cells without the need for feeder cells, providing robust expansion of enriched hNK cells from hPBMCs from qualified donors at day 14 to 15 versus other medium options. Cells maintain CD56+/CD16+/CD3- surface marker expression and demonstrate cytolytic capabilities when expanded in CTS NK-Xpander Medium.
Manufactured for cell therapy applications
CTS NK-Xpander Medium is manufactured without cytokines and growth factors to allow flexibility with regard to intended application. The medium does not include human or animal-derived components; however, supplementation with hAB serum is recommended for best results.
CTS quality
Gibco CTS products follow USP <1043> ancillary materials for cell, gene, and tissue-engineered products within the responsibilities applicable to a supplier.* Documentation such as specific intended-use statements, full documentation traceability, and convenient access to our Drug Master File (DMF) are available to support your regulatory filing.
The products are manufactured at a site that uses methods and controls that conform to cGMP for medical devices, 21 CFR Part 820. Our FDA-registered manufacturing sites are ISO 13485-certified.
Closed-system compatibility
CTS NK-Xpander Medium is available in 500-mL-bottle and 5-L-bag formats for convenient process development and closed-system manufacturing scale-up. The 5-L bioprocess container chamber is constructed from Aegis 5-14 film and includes sterile, weldable DEHP-free PVC tubing with Luer lock and C-Flex™ tubing with MPC quick connector providing versatile connection options for your manufacturing process, including compatibility with the CTS Rotea Counterflow Centrifugation System.
*Other aspects of USP <1043> will be the responsibility of the end-user to assess. Thermo Fisher Scientific cannot fulfill USP <1043> in regards to application and therapy-specific aspects (e.g., residual assessment and removal of the AM).
• Supplement: Store at -20°C and protect from light.