Search
Thermo Scientific Chemicals
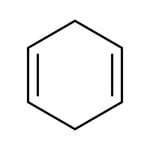
1,4-Cyclohexadiene, 97% stab. with 0.1% BHT
CAS: 628-41-1 | C6H8 | 80.13 g/mol
Catalog number L07337.14
also known as L07337-14
Price (TWD)Request A Quote
-
Quantity:
25 g
Chemical Identifiers
CAS628-41-1
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
Identification (FTIR)Conforms
CommentMaterial sourced in the U.S. and in other countries
Refractive Index1.4710-1.4760 @20°C (non-U.S. sourced material)
Assay (GC)≥96.0%
View more
1,4-Cyclohexadiene is used to study the formation of parent ion from heavy fragmentation on irradiation with a high-intensity laser pulse. It is also used as a very effective hydrogen donor for catalytic hydrogenation reaction. It forms benzene at elevated temperatures in the presence of a ruthenium(II)-triphenylphosphine catalyst.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,4-Cyclohexadiene is used to study the formation of parent ion from heavy fragmentation on irradiation with a high-intensity laser pulse. It is also used as a very effective hydrogen donor for catalytic hydrogenation reaction. It forms benzene at elevated temperatures in the presence of a ruthenium(II)-triphenylphosphine catalyst.
Solubility
Miscible with organic solvents like diethyl ether, tetrahydrofuran and toluene.Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
1,4-Cyclohexadiene is used to study the formation of parent ion from heavy fragmentation on irradiation with a high-intensity laser pulse. It is also used as a very effective hydrogen donor for catalytic hydrogenation reaction. It forms benzene at elevated temperatures in the presence of a ruthenium(II)-triphenylphosphine catalyst.
Solubility
Miscible with organic solvents like diethyl ether, tetrahydrofuran and toluene.Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Mao, J. X.; Mathers, R. T. Damodaran, K. NMR spectroscopic and computational investigations of RuHCl(CO)(PPh3)3 catalyzed isomerization of 1,4-cyclohexadiene. J. Organomet. Chem. 2013, 741-742, 15-19.
- Levandowski, B. J.; Houk, K. N. Theoretical Analysis of Reactivity Patterns in Diels-Alder Reactions of Cyclopentadiene, Cyclohexadiene, and Cycloheptadiene with Symmetrical and Unsymmetrical Dienophiles. J. Org. Chem. 2015, 80 (7), 3530-3537.
- Kakinuma, S.; Shirota, H. Dynamic Kerr Effect Study on Six-Membered-Ring Molecular Liquids: Benzene, 1,3-Cyclohexadiene, 1,4-Cyclohexadiene, Cyclohexene, and Cyclohexane. J. Phys. Chem. B. 2015, 119 (13), 4713-4724.