Search
Thermo Scientific Chemicals
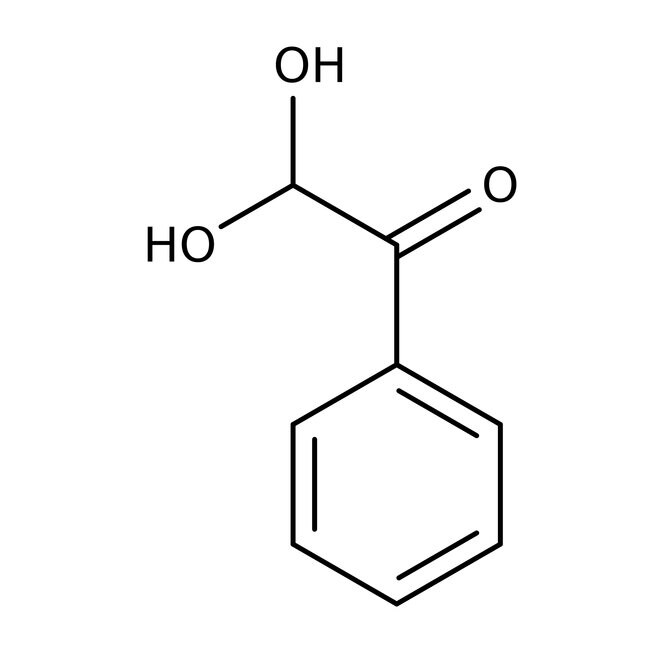
Phenylglyoxal monohydrate, 97%
CAS: 1075-06-5 | C8H8O3 | 152.149 g/mol
| 產品號碼 | Quantity |
|---|---|
| A10677.22 亦稱為 A10677-22 | 100 g |
化學識別
CAS1075-06-5
IUPAC Name2,2-dihydroxy-1-phenylethan-1-one
Molecular FormulaC8H8O3
InChI KeyNBIBDIKAOBCFJN-UHFFFAOYSA-N
SMILESOC(O)C(=O)C1=CC=CC=C1
檢視更多
規格 規格表
規格表
FormCrystals or powder or crystalline powder
Identification (FTIR)Conforms
Water Content (Karl Fischer Titration)6.29-16.77% (0.5-1.5 waters)
Appearance (Color)White to cream to pale brown or pink
Assay (GC)≥96.0%
Phenylglyoxal monohydrate was used to modify pig muscle carbonic anhydraseIII and as a specific reagent for arginine groups. It was also used to prepare pyrrolinone and furan derivatives and as as a chemiluminescent reagent for the determination of purines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenylglyoxal monohydrate was used to modify pig muscle carbonic anhydraseIII and as a specific reagent for arginine groups. It was also used to prepare pyrrolinone and furan derivatives and as as a chemiluminescent reagent for the determination of purines.
Solubility
Partly miscible with water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
Phenylglyoxal monohydrate was used to modify pig muscle carbonic anhydraseIII and as a specific reagent for arginine groups. It was also used to prepare pyrrolinone and furan derivatives and as as a chemiluminescent reagent for the determination of purines.
Solubility
Partly miscible with water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Masaaki Kai; Yosuke Ohkura; Sayuri Yonekura; Masatake Iwasaki. Selective determination of guanine and its nucleosides and nucleotides by reaction with phenylglyoxal as a fluorogenic reagent. Analytica Chimica Acta. 1988, 207, 243-249
- Undergoes the Lewis-acid-catalyzed ene-insertion reaction with simple alkenes. Periodate cleavage of the resulting ɑ-hydroxyketones has been used as a route to ß-unsaturated aldehydes: Synthesis, 413 (1987):