Search
Thermo Scientific Chemicals
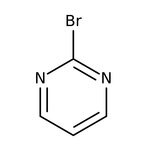
2-Bromopyrimidine, 98+%
CAS: 4595-60-2 | C4H3BrN2 | 158.99 g/mol
化學識別
CAS4595-60-2
IUPAC Name2-bromopyrimidine
Molecular FormulaC4H3BrN2
InChI KeyPGFIHORVILKHIA-UHFFFAOYSA-N
SMILESBrC1=NC=CC=N1
檢視更多
規格 規格表
規格表
Appearance (Color)White to cream or pale yellow or pale brown
Assay (GC)≥98.0% (non-U.S. specification)
Assay from Suppliers CofA≥98.0% (U.S. specification)
CommentPurchased in the U.S. and in other countries
Identification (FTIR)Conforms (non-U.S. specification)
檢視更多
2-Bromopyrimidine is used in the preparation of 2,7-bis(2-pyrimidyl)-9,9-dihexylfluorene by cross-coupling with 9,9-dihexylfluorene-2,7-diboronic acid. It undergoes microwave-assisted aminocarbonylation at C-2 with palladium and molybdenum hexacarbonyl.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Bromopyrimidine is used in the preparation of 2,7-bis(2-pyrimidyl)-9,9-dihexylfluorene by cross-coupling with 9,9-dihexylfluorene-2,7-diboronic acid. It undergoes microwave-assisted aminocarbonylation at C-2 with palladium and molybdenum hexacarbonyl.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents, strong acids, acid chlorides.
2-Bromopyrimidine is used in the preparation of 2,7-bis(2-pyrimidyl)-9,9-dihexylfluorene by cross-coupling with 9,9-dihexylfluorene-2,7-diboronic acid. It undergoes microwave-assisted aminocarbonylation at C-2 with palladium and molybdenum hexacarbonyl.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents, strong acids, acid chlorides.
RUO – Research Use Only
General References:
- Lu, X.; Wang, H.; Gao, R.; Pei, C. One-pot Metal-free Synthesis of Benzyl Alkyl Sulfides. Phosphorus Sulfur Silicon Relat Elem 2015, 190 (1), 45-52.
- Huang, R.; Huang, Y.; Lin, X.; Rong, M.; Weng, Z. Well-Defined Copper(I) Fluoroalkoxide Complexes for Trifluoroethoxylation of Aryl and Heteroaryl Bromides. Angew. Chem. Int. Ed. 2015, 54 (19), 5736-5739.