Search
Thermo Scientific Chemicals
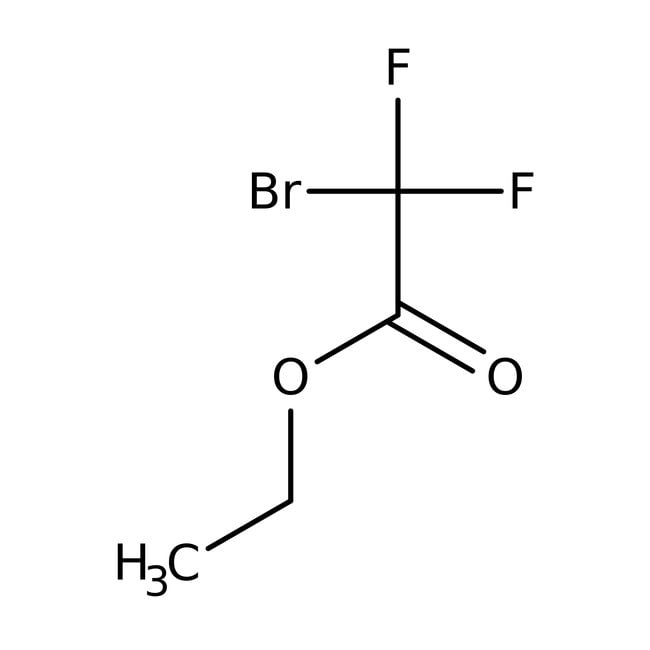
Ethyl bromodifluoroacetate, 97%
CAS: 667-27-6 | C4H5BrF2O2 | 202.983 g/mol
化學識別
CAS667-27-6
IUPAC Nameethyl 2-bromo-2,2-difluoroacetate
Molecular FormulaC4H5BrF2O2
InChI KeyIRSJDVYTJUCXRV-UHFFFAOYSA-N
SMILESCCOC(=O)C(F)(F)Br
檢視更多
規格 規格表
規格表
Assay (GC)≥96.0%
Refractive Index1.3840-1.3890 @ 20?C
Appearance (Color)Clear colorless
FormLiquid
Ethyl bromodifluoroacetate, is used for the treatment of the Reformat sky reagent with aldehydes and ketones affords 2,2-difluoro-3-hydroxy esters. It is also used in the analysis of reagent purity through IR, NMR.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl bromodifluoroacetate, is used for the treatment of the Reformat sky reagent with aldehydes and ketones affords 2,2-difluoro-3-hydroxy esters. It is also used in the analysis of reagent purity through IR, NMR.
Solubility
Soluble in most organic solvents.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away from strong oxidizing agents.
Ethyl bromodifluoroacetate, is used for the treatment of the Reformat sky reagent with aldehydes and ketones affords 2,2-difluoro-3-hydroxy esters. It is also used in the analysis of reagent purity through IR, NMR.
Solubility
Soluble in most organic solvents.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away from strong oxidizing agents.
RUO – Research Use Only
General References:
- S Marcotte.; X Pannecoucke.; C Feasson. Enantioselective Synthesis of α,α-Difluoro-β-amino Acid and 3,3-Difluoroazetidin-2-one via the Reformatsky-Type Reaction of Ethyl Bromodifluoroacetate with Chiral 1,3-Oxazolidines. J. Org. Chem. 199964 ( 23), 8461-8464.
- A Sorochinsky.; N Voloshi.; A Markovsky. Convenient Asymmetric Synthesis of β-Substituted α,α-Difluoro-β-amino Acids via Reformatsky Reaction between Davis' N-Sulfinylimines and Ethyl Bromodifluoroacetate. J. Org. Chem. 200368 ( 19), 7448-7454.
- Valuable building block for the introduction of the CF2 group by Reformatsky reaction with aldehydes. A further development of this chemistry involves the generation of an ɑɑ-difluoro-ß-lactone, followed by thermal decarboxylation as a high-yield route to 1,1-difluoroalkenes: J. Org. Chem., 60, 5378 (1995):