Search
Thermo Scientific Chemicals
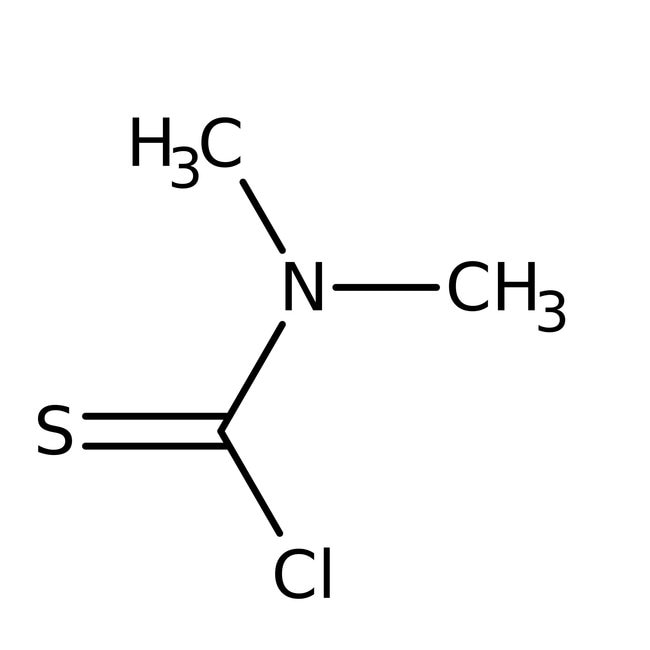
Dimethylthiocarbamoyl chloride, 95%
CAS: 16420-13-6 | C3H6ClNS | 123.60 g/mol
化學識別
CAS16420-13-6
IUPAC Name(chloromethanethioyl)dimethylamine
Molecular FormulaC3H6ClNS
InChI KeyPHWISQNXPLXQRU-UHFFFAOYSA-N
SMILESCN(C)C(Cl)=S
檢視更多
規格 規格表
規格表
Proton NMR94.0% min
Dimethylthiocarbamoyl chloride is used as an intermediate in the synthesis of pharmaceuticals. It finds application in chemoselective deoxygenation of pyridine-N-oxides. It is also used as a starting reagent in the synthesis of dimethylthiocarbamates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dimethylthiocarbamoyl chloride is used as an intermediate in the synthesis of pharmaceuticals. It finds application in chemoselective deoxygenation of pyridine-N-oxides. It is also used as a starting reagent in the synthesis of dimethylthiocarbamates.
Solubility
Soluble in chloroform and tetrahydrofuran.
Notes
Store in cool place. Moisture sensitive. Incompatible with strong oxidizing agents, amines and strong bases. Reacts violently with water.
Dimethylthiocarbamoyl chloride is used as an intermediate in the synthesis of pharmaceuticals. It finds application in chemoselective deoxygenation of pyridine-N-oxides. It is also used as a starting reagent in the synthesis of dimethylthiocarbamates.
Solubility
Soluble in chloroform and tetrahydrofuran.
Notes
Store in cool place. Moisture sensitive. Incompatible with strong oxidizing agents, amines and strong bases. Reacts violently with water.
RUO – Research Use Only
General References:
- Zaim, O.; Tuzun, N. S.; Cevik, B.; Ozcan, H.; Boz, E. Synthesis, reactions and DFT study of tropolone N,N-dimethylthiocarbamate. Tetrahedron 2015, 71 (33), 5391-5398.
- Gandhi, M. R.; Yamada, M.; Kondo, Y.; Sato, R.; Hamada, F. Synthesis and Characterization of Dimethylthiocarbamoyl-Modified Thiacalix[n]arenes for Selective Pd(II)-Ion Extraction. Ind. Eng. Chem. Res. 2014, 53 (7), 2559-2565.