Search
Thermo Scientific Chemicals
Citrate, 0.5M buffer soln., pH 3.0
CAS: 6132-04-3294.10 g/mol
化學識別
CAS6132-04-3
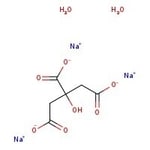
IUPAC Nametrisodium 2-hydroxypropane-1,2,3-tricarboxylate dihydrate
Molecular FormulaC6H9Na3O9
InChI KeyNLJMYIDDQXHKNR-UHFFFAOYSA-K
SMILESO.O.[Na+].[Na+].[Na+].OC(CC([O-])=O)(CC([O-])=O)C([O-])=O
檢視更多
規格 規格表
規格表
CommentBuffer is made by dissolving ingredients in 18.2 megahom, UV treated water, filtered through submicron filter
FormClear Liquid
pH3.0 ± 0.15
Citrate is an intermediate in the TCA cycle and fatty acid synthesis. It is an allosteric modulator of acetyl-CoA carboxylase, the enzyme that regulates the conversion of acetyl-CoA to malonyl-CoA. It has anticoagulant activity and is a calcium chelator. Its function is to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Citrate is an intermediate in the TCA cycle and fatty acid synthesis. It is an allosteric modulator of acetyl-CoA carboxylase, the enzyme that regulates the conversion of acetyl-CoA to malonyl-CoA. It has anticoagulant activity and is a calcium chelator. Its function is to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies.
Solubility
Fully miscible in water. Insoluble in ethanol.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents and heat.
Citrate is an intermediate in the TCA cycle and fatty acid synthesis. It is an allosteric modulator of acetyl-CoA carboxylase, the enzyme that regulates the conversion of acetyl-CoA to malonyl-CoA. It has anticoagulant activity and is a calcium chelator. Its function is to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies.
Solubility
Fully miscible in water. Insoluble in ethanol.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents and heat.
RUO – Research Use Only
General References:
- J.Steigman; G.Meinken; P.Richards. The reduction of pertechnetate-99 by stannous chloride—I. The stoichiometry of the reaction in HCl, in a citrate buffer and in a DTPA buffer. The International Journal of Applied Radiation and Isotopes. 1975, 26,(10), 601-609.
- M.Seruga; M.Metikos-Hukovic; T.Valla; M.Milun; H.Hoffschultz; K.Wandelt. Electrochemical and X-ray photoelectron spectroscopy studies of passive film on tin in citrate buffer solution. Journal of Electroanalytical Chemistry. 1996, 407,(1-2), 83-89.