Search
Thermo Scientific Chemicals
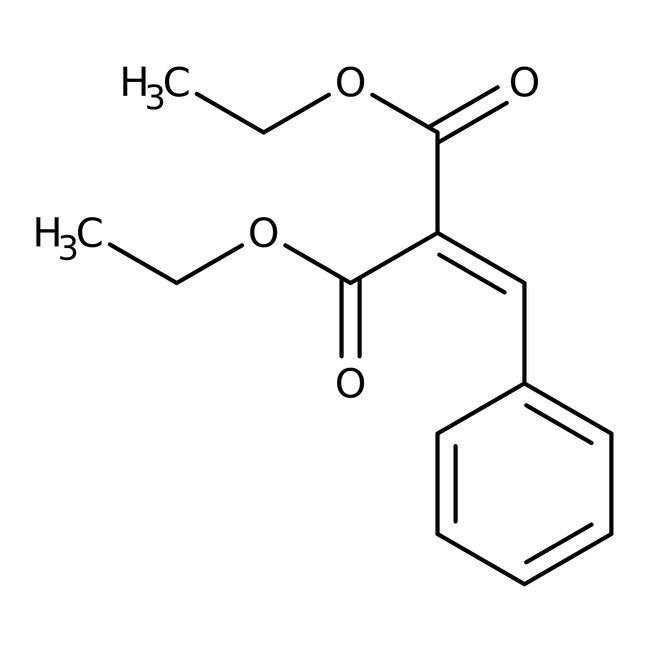
Diethyl benzylidenemalonate, 98%
CAS: 5292-53-5 | C14H16O4 | 248.28 g/mol
化學識別
CAS5292-53-5
IUPAC Name1,3-diethyl 2-(phenylmethylidene)propanedioate
Molecular FormulaC14H16O4
InChI KeyVUWPIBNKJSEYIN-UHFFFAOYSA-N
SMILESCCOC(=O)C(=CC1=CC=CC=C1)C(=O)OCC
檢視更多
規格 規格表
規格表
Appearance (Color)Clear colorless to pale yellow
FormLiquid
Assay (GC)≥97.5%
Refractive Index1.5340-1.5380 @ 20°C
Diethyl benzylidenemalonate is used as a pharmaceutical intermediate. It is also involved in the Conjugate addition of cyanide ion to arylidenemalonic esters provides a useful route to arylsuccinic acids.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diethyl benzylidenemalonate is used as a pharmaceutical intermediate. It is also involved in the Conjugate addition of cyanide ion to arylidenemalonic esters provides a useful route to arylsuccinic acids.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents.
Diethyl benzylidenemalonate is used as a pharmaceutical intermediate. It is also involved in the Conjugate addition of cyanide ion to arylidenemalonic esters provides a useful route to arylsuccinic acids.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents.
RUO – Research Use Only
General References:
- MD Curtis.; P Beak. Asymmetric Carbon-Carbon Bond Formation in Michael Reactions: Conjugate Addition Reactions of Configurationally Stable Benzylic and Allylic Organolithium Species. The Journal of organic chemistry. 199964 (9), 2996-2997.
- P Ivashkin.; S Couve-Bonnaire.; P Jubault. One-Step Synthesis of Highly Functionalized Monofluorinated Cyclopropanes from Electron-Deficient Alkenes. Org. Lett. 201214 (9), 2270-2273.
- Conjugate addition of cyanide ion to arylidenemalonic esters provides a useful route to arylsuccinic acids: Org. Synth. Coll., 4, 804 (1963):