In October 2024, thousands of bottles of the popular antidepressant Duloxetine were recalled due to the presence of N-Nitroso Duloxetine1, a nitrosamine drug substance-related impurity (NDSRI). This issue has continued to pose significant challenges for pharmaceutical manufacturers since nitrosamine impurities were first highlighted in 2018. The ability to accurately measure such impurities is crucial to avoid both product recalls and false positive results, which can prevent essential medicines from being available.

In the realm of analytical chemistry, the precision and accuracy of impurity quantitation are paramount. As pharmaceutical companies strive for higher standards in drug safety and efficacy, selecting the right mass spectrometry technology becomes crucial. Here we delve into a comparative analysis between Thermo Scientific Orbitrap-based technology and perhaps more widely used quadrupole mass analyzers. This analysis highlights the superior performance of the Orbitrap in impurity separation and quantitation, particularly exploring the benefits for detecting N-Nitroso Duloxetine in both Duloxetine drug substance and drug product.
The advantage of Orbitrap technology in impurity analysis
One of the most compelling advantages of Orbitrap technology is its ability to achieve resolutions of up to 500,000 and higher, at the low m/z range which is critical for small molecule profiling. This high resolution enables the separation of ions with very close m/z values, effectively resolving interfering peaks that would otherwise overlap in unit resolution mass spectrometry. Here are some key benefits of this superior resolution:
- Enhanced Peak Separation: In complex mixtures, analytes often exhibit similar m/z values, leading to peak overlap in unit resolution instruments.
- Improved Signal-to-Noise Ratio: High resolution reduces background noise and enhances the signal-to-noise ratio, resulting in cleaner spectra.
- Accurate Isotope Pattern Recognition: Orbitrap’s superior resolution and mass accuracy allow for the precise recognition of isotope patterns, aiding in the determination of molecular formulas and structural elucidation.
- Reduced Chemical Noise: In complex samples, chemical noise can obscure target analytes. Orbitrap’s high resolution minimizes chemical noise, enhancing the detection of trace compounds.
Spotlight on N-Nitroso Duloxetine impurity in Duloxetine drug substance
Conducting a full scan of the API Duloxetine reveals that there is a high-abundant interference peak at a m/z close to the impurity peak of interest (N-Nitroso Duloxetine). To compound the problem, the 13C isomer of the interference peak has only a 0.0083 amu difference from the peak of interest (Figure 1). In comparison, top-of-line quadrupole mass analyzers typically have a resolving power of 0.4 – 0.7 Th, making them unable to distinguish between the 13C isomer of the interfering peak and our peak of interest.
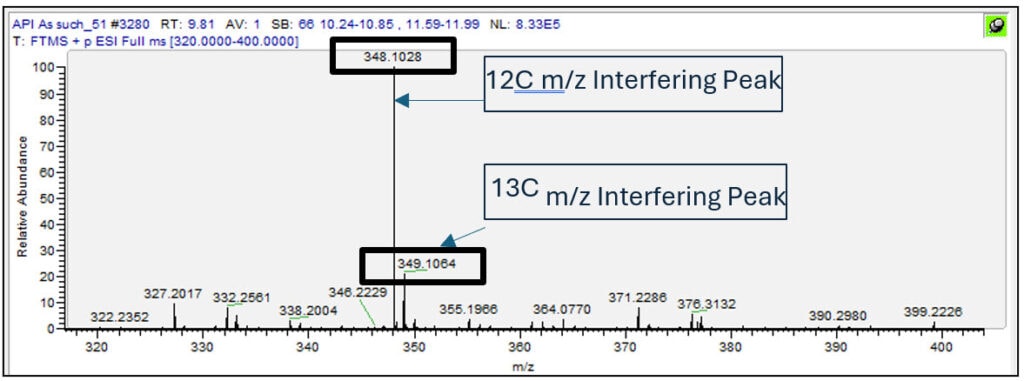

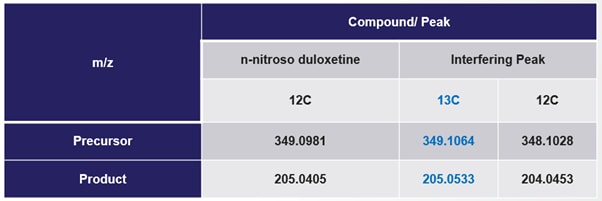
Triple quadrupole mass spectrometry is typically provided with additional selectivity based on selective monitoring of specific fragmentation ions of the selected precursor ion, however in this case the interfering peak also has very similar product ions following dissociation (table 1).
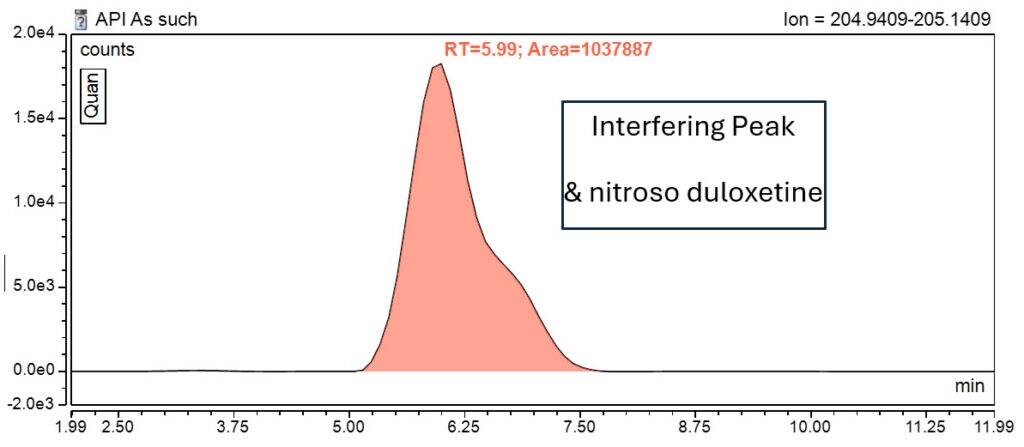
This makes it difficult to distinguish between the two species, leading to an overestimation of the N-Nitroso Duloxetine impurity (Figure 2 and Table 2).

What is the impact of the data?
In this case, the overestimation of the impurity N-Nitroso Duloxetine would have been significant enough to halt production and initiate an investigation. Worse still, it could have led to an unnecessary batch recall, incurring substantial costs for the manufacturer.

By utilizing Orbitrap technology in manufacturing release tests, the impact is two-fold:
- Greater confidence in Drug Safety and Efficacy: This technology ensures that the drug on market is safe and maintains its efficacy. Additionally, it helps to avoid unnecessary financial impacts due to false positive results, which could otherwise lead to unwarranted production halts or batch recalls.
- Efficiency in Analysis: The complexity of the samples analyzed can lead to extensive method develop times, and long analysis durations. Implementing Orbitrap technology reduces both, by complementing chromatographic separation with the high resolution and mass accuracy of Orbitrap acquisition. This efficiency not only speeds up the analytical process but also enhances the reliability of impurity detection and quantification.
Orbitrap mass spectrometry represents a paradigm shift within the GMP environment. Its ability to resolve interfering peaks with high precision makes it an invaluable tool for researchers and analysts working with complex samples. While unit resolution mass spectrometry has its place, the superior performance of Orbitrap technology allows for the tackling of difficult analytical challenges that could lead to costly implications for drug manufacturers.
Orbitrap mass spectrometers, supported by compliance-ready Thermo Scientific Chromeleon CDS software, are currently implemented in multiple global manufacturing companies within fully compliant environments and are already paying dividends to those who have adopted the technology in this market, where data integrity is of upmost importance. As the challenges of drug impurity testing become more complex, investing in the appropriate tools will help you navigate this complexity and ultimately reduce operational costs associated with false results.
Operational simplicity
Discover how simple it is to run the orbitrap mass spec and scale up your LC-MS experiment with confidence by downloading our content pack, exploring how the Orbitrap Exploris meets your needs for efficient analysis through short, instructional videos on operation and maintenance.
References
- USP nitrosamine exchange community https://nitrosamines.usp.org/t/n-nitroso-duloxetine-recall-back-in-the-news/11690