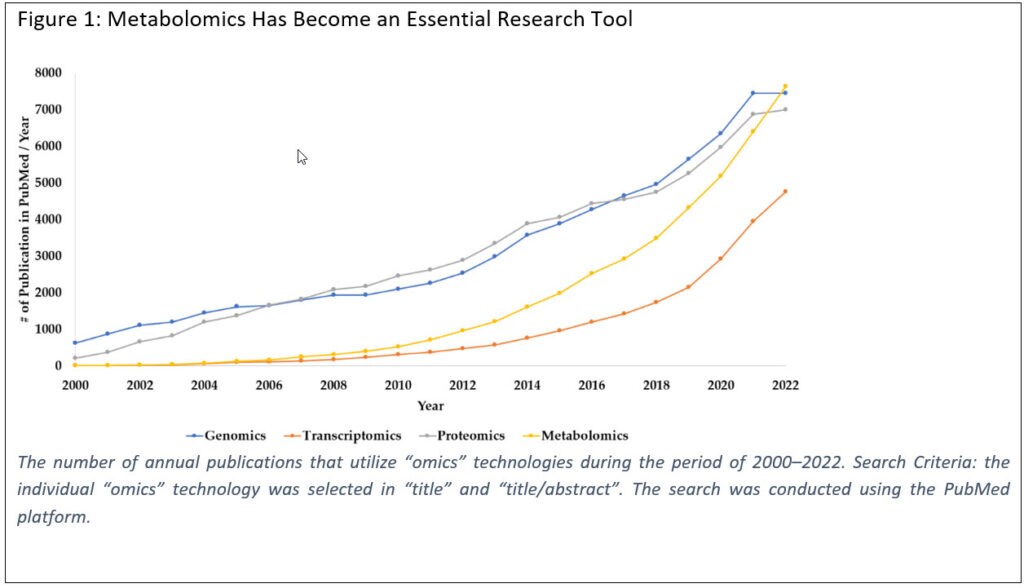
In the last two decades, metabolomics, a member of the “omics” family, has become an essential tool in many areas of research (Figure 1), involving medicine, biology and agriculture, among others. But, for metabolomics to provide valuable biological insights and robust reproducible measurements, confident metabolite identifications are required. To help improve the overall quality and reproducibility of datasets, the metabolomics community is recognizing the need for defined quality assurance (QA) and quality control (QC) in metabolomics.
Why Prioritize QA/QC for Metabolomics Now?
Metabolite analysis heavily utilizes liquid chromatography mass spectrometry (LC-MS); however, the technological advances of LC-MS have not fully translated to metabolomics applications. Innovation in MS technology for metabolomics has remained arguably stagnant until recently, when a novel single-injection metabolomics method was introduced.

Simultaneous quantitation and discovery (SQUAD) analysis is a single-injection metabolomics method introduced to identify and accurately quantify a targeted set of metabolites, while also capturing untargeted discovery data with minimal compromise. With the ability to analyze all untargeted metabolomics, all measurable metabolites can potentially be uncovered in a biological sample. But, to effectively apply SQUAD and ensure the reliability and accuracy of metabolomics data, it is essential to have a robust quality assurance and quality control program in place.
How to Implement Metabolomics QA/QC in Your Lab
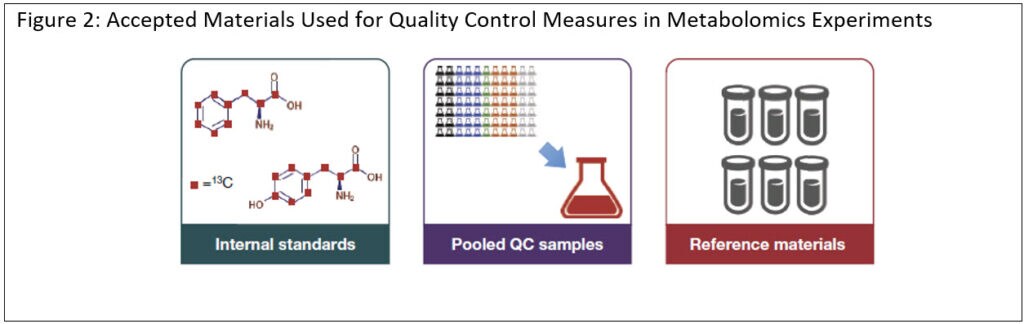
Discover a QA/QC framework for maintaining the quality of data generated during metabolomics experiments in this technical note. The QA/QC program we outline details the processes we employ, not only in Thermo Fisher labs but also with other scientists, to bring a next-generation level of integrity to metabolomics data quality and robustness.
With QA/QC in place, your lab will be better equipped to improve the integrity and robustness of untargeted metabolomics datasets and save time and valuable samples by obviating the need for repeated analysis.
Join us in advancing metabolomics research: establish an effective quality program for your analytical lab. Read the technical note.

Quality assurance and quality control in metabolomics: achieving high-quality data for high-quality results
Authors: Bashar Amer, Rahul R. Deshpande, Amanda Souza, and Susan S. Bird
Thermo Fisher Scientific, San Jose, Calif.



