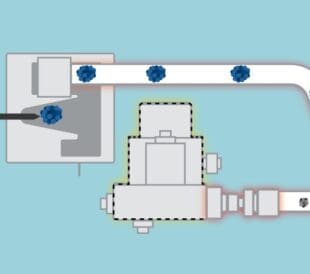
Welcome back to our series on Charged Aerosol Detection (CAD) in High-Performance Liquid Chromatography (HPLC). In the first two episodes, we explored the fundamentals and benefits of CAD and compared it to other universal detectors like ELSD and MALS. In this episode, we will delve deeper into the inner workings of CAD, providing a detailed understanding of its operation and the technology behind it.

The basics of CAD
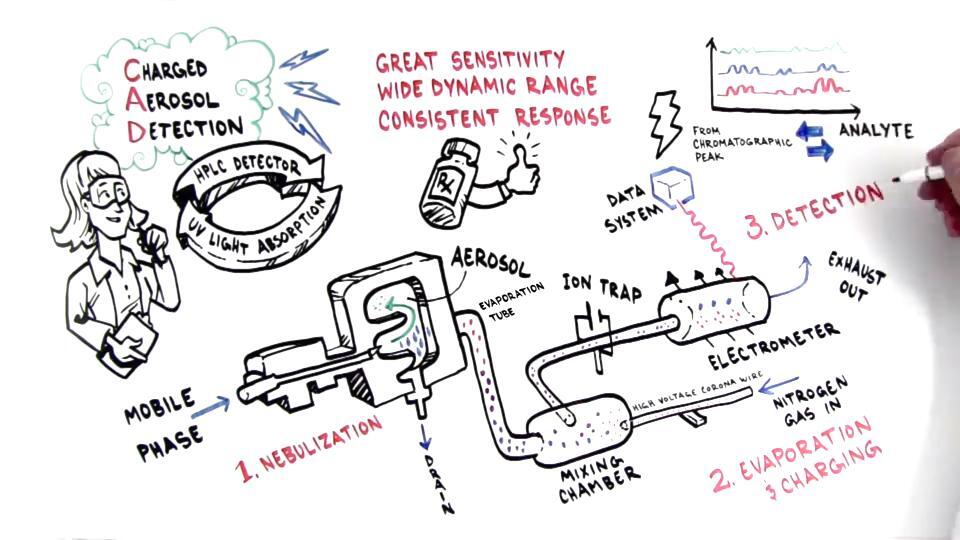
Charged Aerosol Detection (CAD) is a universal detection method used in HPLC that provides a consistent response for non-volatile and semi-volatile analytes. CAD operates by converting the liquid eluent from the HPLC column into charged aerosol particles, which are then detected to generate a signal proportional to the mass of the analyte. Let’s break down the process step by step.
Step-by-step operation of CAD
1. Nebulization
The first step in CAD is the nebulization of the liquid eluent from the HPLC column. The eluent is introduced into a nebulizer, where it is transformed into a fine aerosol. This is achieved by using a high-velocity gas stream (usually nitrogen) that shears the liquid into tiny droplets. The size of these droplets is typically in the micron range.
Key components:
- Nebulizer: Converts the liquid eluent into an aerosol.
- Gas stream: Typically nitrogen, used to create the aerosol droplets.
Detailed process: The liquid eluent enters the nebulizer, where it meets a high-velocity gas stream. This interaction causes the liquid to break up into small droplets, forming an aerosol. The efficiency of nebulization is crucial, as it determines the size and uniformity of the droplets, which can affect subsequent steps in the detection process.

2. Evaporation
After nebulization, the aerosol droplets enter a heated drift tube. In this tube, the solvent evaporates, leaving behind dried analyte particles. The temperature of the drift tube is carefully controlled to ensure complete evaporation of the solvent without decomposing the analyte.
Key components:
- Heated drift tube: Evaporates the solvent, leaving dried analyte particles.
Detailed process: The aerosol droplets pass through the heated drift tube, where they are subjected to a controlled temperature. The solvent in the droplets evaporates, resulting in the formation of dried particles that contain the analyte. The drift tube’s temperature must be optimized to prevent the degradation of thermally sensitive analytes while ensuring complete solvent removal.
3. Charging
The dried analyte particles then pass through a corona discharge, where they are exposed to a high-voltage electrical field. This field imparts a charge to the particles. The corona discharge generates ions that attach to the analyte particles, effectively charging them.
Key Components:
- Corona discharge: Generates ions to charge the analyte particles.
Detailed process: As the dried particles exit the drift tube, they enter the corona discharge region. Here, a high-voltage electrode ionizes the surrounding gas, creating a corona discharge. The ions generated in this discharge attach to the analyte particles, imparting a charge to them. This charging process is critical, as the strength of the signal detected later is proportional to the charge on the particles.
4. Detection
The charged particles are then directed into a detector, typically an electrometer, which measures the quantity of charge. The signal produced by the electrometer is proportional to the amount of analyte present. This signal is then processed and displayed as a chromatogram, showing the concentration of the analyte over time.
Key components:
- Electrometer: Measures the charge on the analyte particles.
- Data processor: Converts the measured charge into a readable signal.
Detailed process: The charged particles flow into the electrometer, where the charge is measured. The electrometer converts the charge into an electrical signal, which is proportional to the quantity of analyte. This signal is then processed by data analysis software, which generates a chromatogram. The peaks in the chromatogram correspond to the concentration of the analyte at different time points, allowing for quantitative analysis.
You can find more information on why to choose Charged Aerosol Detection for your HPLC analysis
Conclusion
Charged Aerosol Detection (CAD) is a powerful and versatile detection method in HPLC, offering consistent response, high sensitivity, robustness, and ease of use. By understanding the step-by-step operation of CAD, we can appreciate the technology’s ability to provide reliable and accurate detection across a wide range of analytes.
As we continue our series on CAD, stay tuned for the next episode, where we will explore specific case studies and practical applications of CAD in various industries, demonstrating its real-world impact and benefits.
Thank you for joining us in this deep dive into the workings of Charged Aerosol Detection. We hope this episode has provided you with a clearer understanding of CAD and its advantages in analytical chemistry.
Visit us on LinkedIn: #CAD, #ChargedAerosolDetection #HPLC