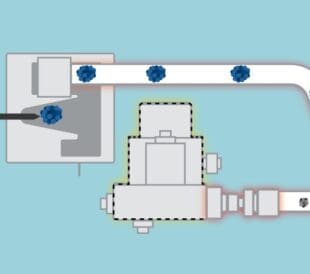
Extractables and leachables (E&L) in liquid solutions are ubiquitous and unavoidable culprits. Even more difficult, the compounds sometimes take on unexpected chemical forms that are hard to detect and identify.
The Charged Aerosol Detector (CAD) is highly suited for the analysis of complicated, chromophore-deficient, potentially toxic plastic oligomers that extract into solution. Because charged aerosol detection works by detecting aerosolized particles, the response is independent of the chemical structure, meaning you can also see compounds that lack double bonds (UV-Vis) or cannot ionize (mass spectrometry/MS).
Now, given the structural versatility of extractable polymers, you’ll likely want to consider using a multidetector approach. By combining CAD + UV + MS detection, your analytical method transforms into a powerful tool for the thorough characterization of chemically complex samples.
Here’s how: With near-universal detection and surrogate-standard quantitation, the CAD enables you to detect and quantify all non-volatile and many semi-volatile known and unknown compounds. The UV can reveal trace levels of unknowns with strong chromophores. The MS data allows you to confirm the identity of unknowns seen in the CAD and UV data.
Let’s explore a real-life example.
A multidetector method for characterizing oligomers extracted from Nylon 6 burn wound dressing
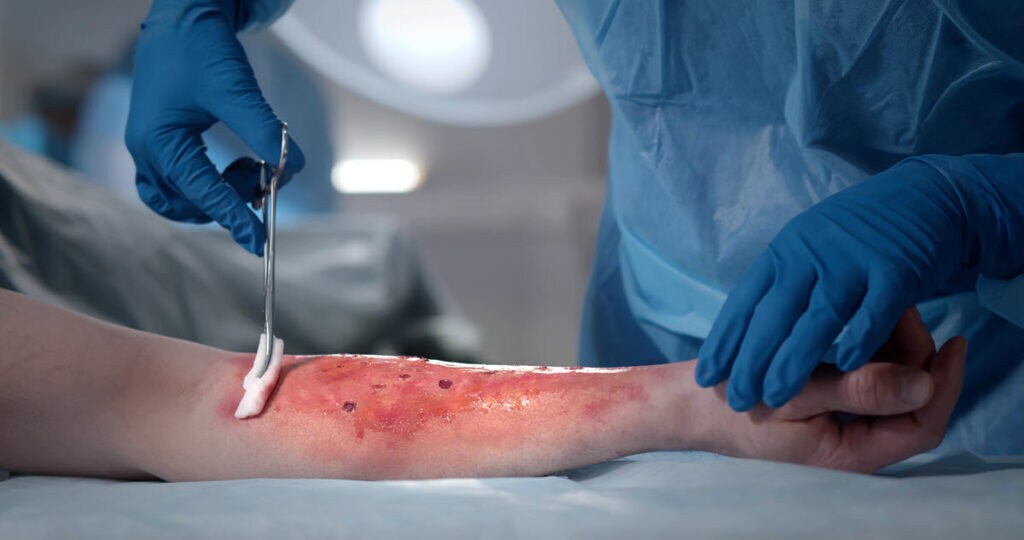
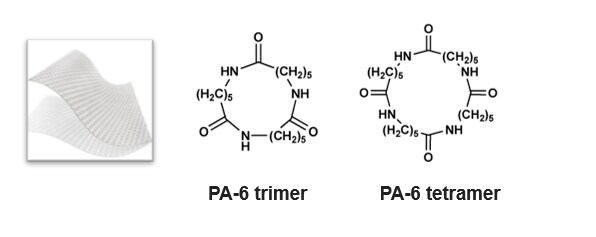
Cyclic oligomers of the caprolactam monomer (Figure 1) that comprise Nylon 6 are potentially hazardous to your health, depending on the amount, especially when delivered directly into your body at the site of a deep burn wound. Understanding the extractable concentration of Nylon 6 oligomers in burn wound dressings is critical for patient safety.
The CAD is well-suited for the analysis of nylon oligomers, which lack chromophores and multidetector analyses are published by BMW and others. In this experiment, the wound dressing was extracted for 72 hours with 50/50 isopropanol/water and then analyzed for plastic oligomers.
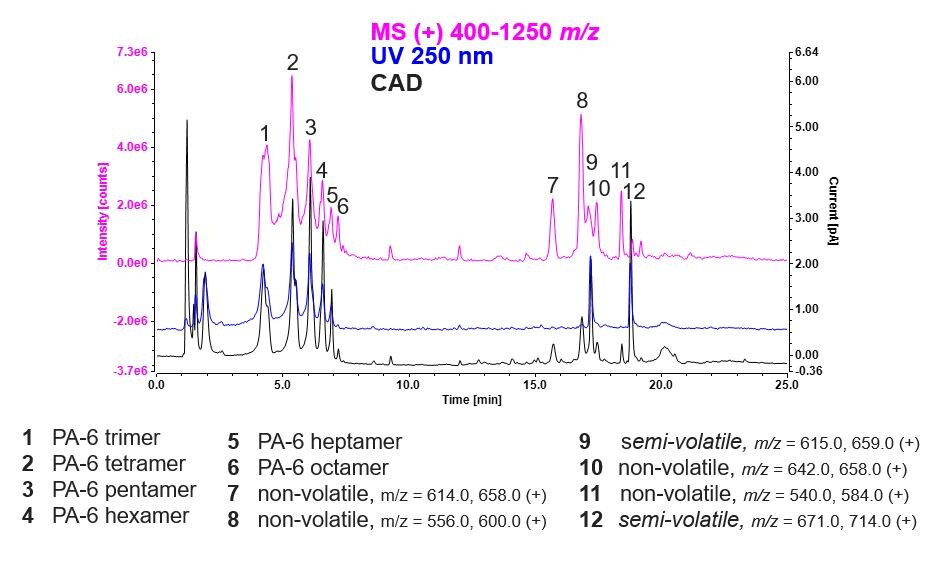
The chromatograms presented in Figure 2 show an overlay of the CAD-UV-MS traces post-extraction. The CAD chromatogram (black) was used for quantitation, and the UV trace (blue) at 250 nm is for the same sample. The MS trace (pink) for the same sample, was used to confirm the identify of unknowns. When compared with a mass list of potentially hazardous nylon extractables, a number of polyamides, from the trimer to the octamer, are tentatively identified.
One thing to note is the individual calibration of most oligomers is generally impossible because standards are not commercially available. With the CAD, you can accurately quantify unknown and unexpected non-volatile substances with a surrogate standard (Irganox 245).
How to assign unknowns as CAD semi-volatiles or non-volatiles
Changing the evaporation temperature (EvapT) of the CAD is a strategy you can use for confident assignment of unknowns as semi-volatiles or non-volatiles (Figure 3). The black chromatogram is the CAD trace from Figure 2. The green overlay is the same sample analyzed with an elevated EvapT.
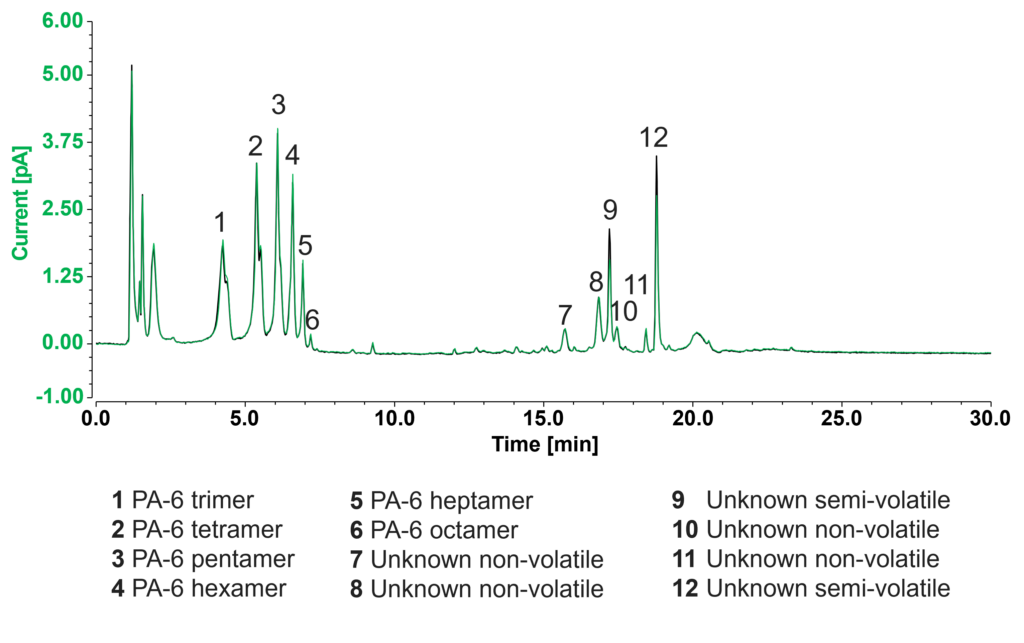
Any peak that has roughly the same area at the higher EvapT will have approximately the same calibration curve as any other non-volatile substance. In this sample, 10 of 12 unknowns have the same area at 35 °C and 50 °C and all can be quantified by the calibration curve of the surrogate standard (Irganox 245).
Unknown peaks that fail the CAD non-volatility test are good candidates for less accurate quantification by LC-MS or GC-MS.
How can you apply this multi-detection method to other E&L analyses?
Our new application note provides details of a routinely used, in-house method for E&L quantification and for single-quadrupole mass spectrometer library data generation. We can classify unknowns as non-volatiles or semi-volatiles in the CAD and quantity non-volatile unknowns using a universal calibrant, even when they could not be identified. We also improved quantification accuracy and reduced response factor variation using an inverse gradient and showed best practices in eliminating baseline drift in the CAD.
The combination of CAD, MS and UV saves sample and time by allowing for:
- Accurate quantitation of non-volatile unknowns by CAD (%RSD within 15%)
- Identity confirmation of unknowns with an easy-to-operate single quadrupole MS
- Detection of UV-absorbing trace contaminants in a single injection.
Explore our collection of white papers and see how HPLC-CAD methods can benefit your pharmaceutical analyses here.