Struggling to accurately measure semi-volatile analytes? Stricter temperature control with HPLC-CAD analysis just might be the solution you need.

The charged aerosol detector (CAD) is cited as one of the most capable near-universal HPLC detectors available because the response is solely based on particle charge and fully independent of chemical structure.
One caveat to know is your mobile phase components must be less volatile than the analytes in solution for detection by the CAD. And while all non-volatiles give a similar uniform response, the response of semi-volatiles depends on volatility and consequently is less predictable.
So, the question then becomes is there a way to address this performance difference and make semi-volatile detection more predictable? One approach for charged semi-volatile analytes is to add a volatile ion-pairing agent of the opposite charge in the mobile phase to force non-volatile behavior.
However, this method is analyte specific and may also react with impurities in your mobile phase, leading to higher noise and poorer signal-to-noise (S/N) ratio. What’s really needed is a more global approach to improve the accuracy for measuring semi-volatiles.
This approach is now achievable with Temperature Coupling Mode (TCM) in the Thermo Scientific Vanquish CAD P Series.
Why stricter temperature control matters for measuring semi-volatiles
Before we get into the specifics, you need to understand why temperature control in the CAD is critical to quantitative accuracy, especially for semi-volatiles (Figure 1).
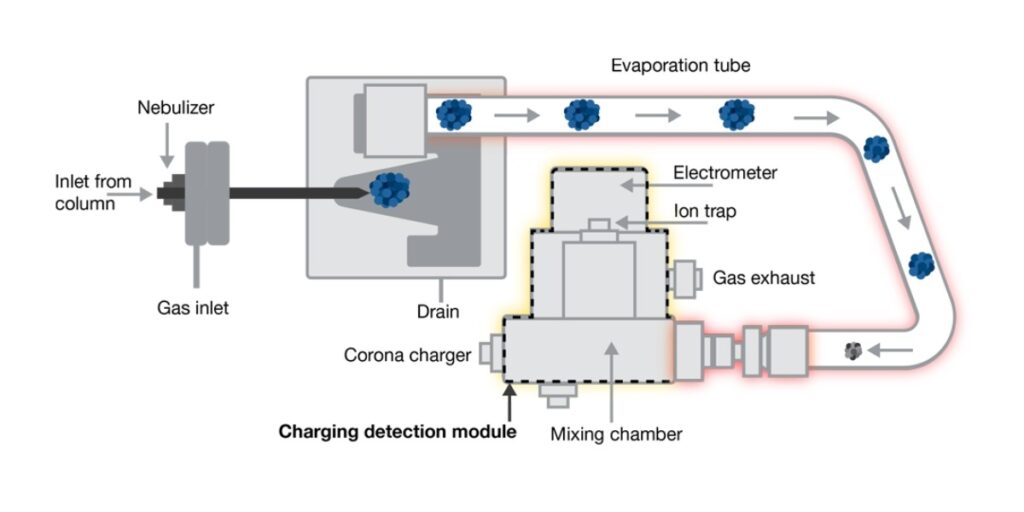
Figure 1. Illustration showing how the CAD works.
The two most important functional components in the CAD that guide sensitivity are the evaporation tube temperature (EvapT) and the charging detection module (CDM).
- EvapT plays a crucial role in determining the performance of the detector, and changing this temperature may alter analyte “selectivity”
- The CDM is where particle charge and measurement happen
In previous CAD models, the EvapT is adjustable, but the temperature of the CDM is permanently set at 40 °C. This fixed temperature difference between the EvapT and CDM could negatively impact the measurement of semi-volatile compounds that require a lower EvapT.
Adding TCM resolves this issue, by linking the EvapT and CDM temperatures, thereby improving the quantitation of semi-volatile species.
Using TCM to help improve quantification of semi-volatiles, like fatty acids
To demonstrate the effectiveness of this new temperature coupling feature, we anlazyed a working solution of four individual fatty acids (FAs) and compared the CAD repsonse with TCM turned on and turned off (Figure 2).

Figure 2. The mean S/N ratio of the FAs analyzed with TCM turned on and off.
Notably, we observed 46.8% increase in the S/N at the optimal EvapT of 25 °C and with the TCM switched on for lauric acid, the most semi-volatile compound!
Interestingly, the observed S/N improvement matches the respective analyte volatility—lauric acid (lowest MW) shows the greatest improvement, and stearic acid (highest MW) the least.
Taken together, these findings show how stricter temperature control in the CAD can allow you to extend the range and improve quantification of semi-volatiles.
Extending this application to impurity analysis of polysorbates
Now that we’ve seen TCM is useful for analyzing fatty acids, let’s consider more complex solutions, like polysorbates.
Given the CAD is well-established for determining polysorbate impurities, and fatty acid impurities are quite abundant in polysorbates, and a logical next step is to use this technique for analyzing these solutions.
Afterall, free acids in polysorbates may form insoluble particles that can seriously compromise product quality, efficacy, and patient safety. Thus, your ability to accurately measure these acids in raw materials and finished goods is of the upmost importance.
Download our paper to see how the Vanquish CAD P Series can help your lab measure fatty acids with greater confidence today.

Figure 3. Thermo Scientific Vanquish CAD P Series
FAQs
- What are the advantages of using a charged aerosol detector for HPLC analysis?
The charged aerosol detector (CAD) is now recognized as the most capable “universal” detector available and offers several advantages over other UHPLC-based detectors including:
- Uniform response for all non-volatile analytes (ideal for quantitation when certified standards are unavailable)
- Universal response independent of chemical structure (analyte does not have to form gas phase ions or possess a chromophore)
- High sensitivity at high pg to low ng on column (ideal for drug impurity analysis)
- Wide linear and dynamic range (measure API and impurities in one run)
- Simple calibration curves (no need to deal with complex, unpredictable sigmoidal calibration curves)
- Gradient compatible (inverse gradient available to ensure consistent response throughout the gradient)
2. Why should I use CAD for measuring fatty acid impurities in polysorbates?
Fatty acids don’t contain strong chromophores, so HPLC-CAD analysis is the ideal choice since the detector response depends on particle charge and is independent of analyte structure.
3. How does changing the evaporation tube temperature affect the CAD response for semi-volatiles?
A high EvapT can improve sensitivity for non-volatiles by reducing noise but may negatively impact measurement of semi-volatile species. Conversely, a lower EvapT can improve the detection of semi-volatile species, but sensitivity for all analytes may be adversely impacted due to increased noise.
Visit us on LinkedIn: #HPLC #CAD #biopharma