The discovery of stem cells accelerated the progress of many numerous new fields in biology and biotechnology. Capable of differentiating into numerous cell types, they provide everything from the base material needed for regenerative medicine applications, including platforms for cell line generation and drug discovery testing. But what are stem cells, and what does it take to work with them in their powerful pluripotent state?
What is a Stem Cell? 
Stem cells are undifferentiated cells that, through the activation of specific genes and pathways, can turn into other cell types when they divide. They play a key role in the growth and development of multicellular organisms. In animal embryos, they are the base material for building new tissues and organs. Pluripotent stem cells, which can turn into any other kind of cell, are primarily present during the early stages of embryo development, whereas many kinds of adult or multipotent stem cells continue to exist throughout an animal’s life, used to regenerate blood, skin, and other tissues as part of healing and renewal processes. Adult stem cells are much more limited than pluripotent stem cells. Adult stem cells typically can differentiate only into a small subset of tissues, such as blood cells (which start as adult stem cells in bone marrow) rather than the much wider array of possible futures that pluripotent stem cells can have.
Harvesting adult stem cells is a difficult and sometimes fraught endeavor, but there is an alternative. With clever molecular reprogramming techniques that manipulate transcription factors, a somatic cell can be induced to become a stem cell. These induced pluripotent stem cells have become the backbone of stem cell technology and research today.
The applications of pluripotent stem cells are as numerous as they are diverse. Stem cells can be model systems for studying normal tissue development, they can generate ultra-specific disease-relevant cell types to understand specific disease pathologies, they can serve as test subjects for experimental drugs; and theoretically can even generate fresh tissue on demand to replace tissue lost to injury or disease.
All of this promise requires a workflow that can address the special challenges that stem cells impose. And that’s where Gibco comes in.
Steps to Success
There are many steps to turning somatic cells into a stem cells and their subsequent use in disease modeling, regenerative medicine or manufacturing pipelines.
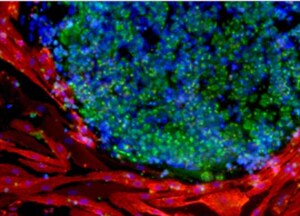
Infrared image of a reprogrammed PSC colony
First, the somatic cells are cultured and reprogrammed into induced pluripotent stem cells by forcing the expression of several reprogramming factors.
Following the establishment of an induced pluripotent stem cell line, those stem cells must be further cultured and expanded to ensure there are enough of them for downstream steps. During the expansion of these induced pluripotent stem cells, the cells are characterized to ensure their pluripotency status and the lack of any genomic abnormalities that may accumulate during the reprogramming or culture steps.
Depending on one’s goals, it might be necessary to engineer the stem cells to ensure they have certain properties or genetic characteristics. This is done with further genetic engineering tools, including the powerful, customizable CRISPR-Cas9 enzyme complex.
Between engineering the stem cells and turning them into the final cell type/tissues (differentiation), stem cells must usually be recovered and cryopreserved. The results of genetic engineering can often only be observed and measured once the stem cells are allowed to differentiate into the desired somatic cells of interest, such as neurons, muscle cells (cardiomyocytes), or immune cells.
All of these steps require specialized instruments, reagents, and expertise, and often different versions thereof, depending on what kind of pluripotent stem cells one is using, the original model organism, and whether one is operating from a research, biomedical, or manufacturing context.
The GibcoTM CTSTM portfolio of cell and gene therapy products is manufactured in GMP-compliant settings, safety tested, and backed by regulatory documentation to support the whole sequence of induced pluripotent stem cell research, from somatic cell culture all the way to differentiation and measuring final experimental results. Gibco offers instrumentation, software, consumables, and dedicated testing, regulatory, and quality control support for every step in the stem-cell research process. Gibco also offers online videos, e-learning resources, product guides, and handbooks to guide new researchers on the way to becoming experts in this challenging field.
Have a look at our catalog and web resources today to find out how to get started with induced pluripotent stem cell research.
Related links:
Co-culturing patient-derived cancer organoids with fibroblasts and endothelial cells
Making Proteins with Expression Workflows
Leave a Reply