
The 2020s is the era of recombinant antibody production and therapeutic proteins – including monoclonal antibodies, bispecifics, Fc fusions – which account for almost 10% of the total global market of prescription drugs today.1 Therapeutic pipelines in 2025 are flush with recombinant proteins designed to treat a wide range of indications: cardiovascular disease, chronic respiratory syndromes, autoimmunity, diabetes, multiple types of cancer, and more. 2–6
As demand for this promising drug class grows, so does the question about how to scale and improve complex drug discovery workflows: to buy or to build? Many of today’s biopharma organizations are finding that outsourcing key steps of pre-clinical discovery like antibody expression offers cost, time, and quality advantages over in-house investment.
This overview breaks down why so many are choosing to innovate with the help of recombinant antibody production services.
Table of contents
- What are the steps of recombinant antibody production?
- Common approaches to antibody optimization before expression
- Five key benefits of using a recombinant antibody production service
- Potential drawbacks: should you always outsource antibody service?
- Recombinant antibody production services can help speed discovery.
- References
What are the steps of recombinant antibody production?
Recombinant antibody production is a complex and specialized process involving five main phases of work:
- Selecting the target antigen: A molecule of interest (antigen), like a tumor receptor, is identified and characterized to help ensure it can trigger an immune response and serve as a target for antibody development.
- Creating an antibody library: A diverse collection of antibody sequences is generated, often using tools and techniques like synthetic gene libraries or phage display, to maximize the chances of finding a high-affinity binder.
- Screening for high-affinity antibodies: The antibody library is screened using high-throughput methods to isolate antibodies that strongly and specifically bind to the target antigen.
- Engineering and optimizing recombinant antibody properties: Selected antibodies undergo modifications like affinity maturation or humanization to improve stability, specificity, and therapeutic potential.
- Expressing and purifying antibodies: Optimized recombinant antibody sequences are expressed in host cells (e.g. mammalian or bacterial systems), and the antibodies are purified to help ensure high quality and functionality for research or therapeutic use.
Common approaches to antibody optimization before expression
The engineering and optimization step of recombinant antibody production represents an ambitious challenge on its own. Through any number of structural and chemical design choices, drug developers can modify intended therapeutic functions, pharmacokinetics, and beyond.
Affinity maturation strategies, for example, can improve target binding affinity and specificity.7 Isotype selection can improve efficacy and tolerability, and specially engineered construct enhancements can optimize immune effector functions.8 Recombinant protein modifications directed at Fc neonatal receptor (FcRn) interactions can lead to better half-life and biobiodistribution.9
Antibody engineering
Five of the most common engineering techniques include:
- Antibody Humanization – The process of modifying a non-human antibody (e.g., from mice) to retain its antigen-binding ability while replacing most of its structure with human antibody sequences to reduce immune rejection in therapeutic use.
- Affinity Maturation – An optimization technique that introduces mutations in the antibody’s binding region to enhance its strength and specificity for the target antigen, improving its efficacy.
- Bispecific Antibody Production – The engineering of antibodies that can bind to two different antigens or two distinct epitopes on the same antigen, enabling novel therapeutic mechanisms such as bringing immune cells and tumor cells together.
- Antibody Fragment Engineering – The design of smaller antibody fragments (e.g., Fab, scFv, nanobodies) that retain antigen-binding properties while improving tissue penetration, stability, or reducing immunogenicity.
- Antibody Fusion – The attachment of an antibody or antibody fragment to another molecule, such as an enzyme, toxin, or drug, to enhance therapeutic effects by delivering targeted treatments to diseased cells.
Using these strategies and more, the potential for combined, performance-enhancing construct modifications is nearly endless.
DNA sequence optimization
DNA sequence optimization adds another key layer for process enhancement in achieving a lead antibody candidate. By using favorable codons – specific to the gene expression system – and removing sub-optimal sequences, it is possible to significantly increase final expressed yields without loss of protein function.
Algorithmic optimization tools like Invitrogen™ GeneArt™ GeneOptimizer™ software automate the complex, multi-parameter analyses that determine which sequence factors can promote excellent protein expression levels. Through sequence optimization methods, today’s drug researchers can quickly refine transcription, splicing, translation, mRNA degradation, and more in their gene design towards novel therapeutic proteins.

Challenges of recombinant antibody production
Endless protein optimization prospects present exciting playgrounds for innovation, but that potential can be a double-edged sword for R&D experts. For workflows to keep up, processes need to accommodate the sheer number of possibilities – screening many thousands of antibody candidates in a reasonable time frame and expressing the leading candidates at scale.
Moving from a target to a lead antibody candidate – from screening step and on – represents a major bottleneck in the industry.
High-throughput antibody discovery services and platforms, which offer specialized resources for the rapid production of candidate antibodies from transiently transfected mammalian cells, emerged as one answer to the challenge of overwhelming discovery volume. In 2023, these services were valued at more than 1.6 billion dollars.10
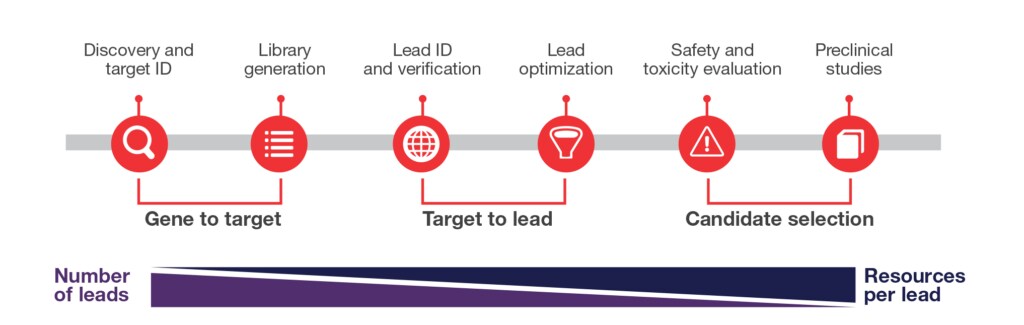
Five key benefits of using a recombinant antibody production service
For biotech and pharma companies, outsourcing antibody expression services to specialized partners has emerged as a strategic advantage in the numbers game of “failing fast” towards success. But is this the right move for every company?
Below are five benefits to consider.
1. Time to market
Speed is a critical factor in drug development, where a first-to-market advantage can translate into billions in revenue. Research shows that delays can cost approximately $500,000 per day in unrealized drug sales, while direct daily clinical trial expenses average $40,000.
With such high stakes, rigid development timelines leave little room for setbacks. Obstacles in antibody expression can result in missed clinical trial milestones, regulatory deadlines, and reporting requirements—wasting valuable time and resources.
Outsourcing to specialized antibody expression service providers helps mitigate these risks. With optimized workflows, high-throughput platforms, and deep regulatory expertise, these partners can compress development timelines from months to weeks. For example, GeneArt services offer custom antibody expression with a turnaround of 20 business days or fewer, helping to ensure that researchers stay on track and maintain momentum.
2. Cost efficiency
Establishing in-house antibody expression capabilities demands a significant capital investment in infrastructure, reagents, and skilled personnel. For many companies, outsourcing helps to provide a cost-effective alternative, freeing up resources to focus on critical areas like clinical trials and regulatory approvals.
Partnering with an experienced expression services provider can help with the logistical side, too. GeneArt services match clients with dedicated technical project managers to help guide project setup and communication.
3. Access to advanced technology and niche expertise
Service partners that specialize in antibody expression often prioritize always-up-to-date access to the AI-driven optimizations, high-throughput screening tools, and automated technologies.
Some providers offer helpful technologies. For example, GeneArt HTP Antibody Expression Services includes access to GeneOptimizer, the most advanced gene optimization algorithm on the market. GeneArt protein expression services also use the highly-cited Gibco™ ExpiCHO™ and Expi293™ transient expression systems – developed by long-time Thermo Fisher Scientific R&D scientist Dr. Henry Chiou.
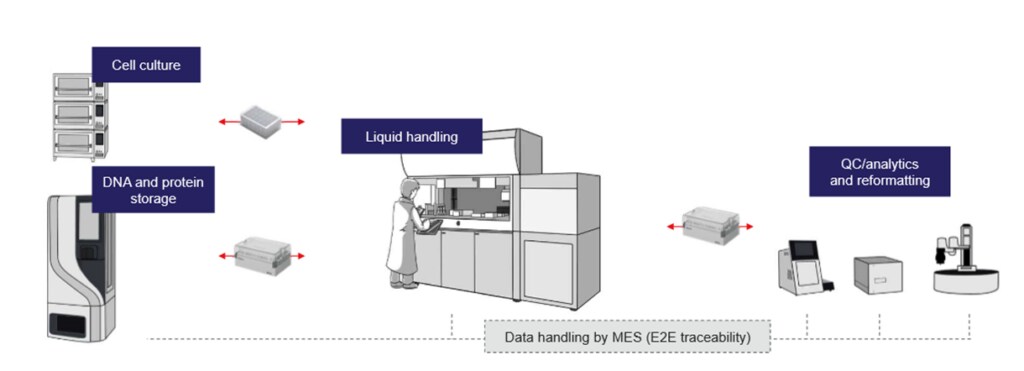
4. Scalability and flexibility
Biotech startups and large pharma alike benefit from on-demand scaling—whether it’s small-batch production for early-stage research or large-scale biomanufacturing for commercialization. This flexibility is crucial in a rapidly evolving landscape where project needs can change overnight.
With dedicated high-throughput processes in place, specialized service providers can also set the stage for future scaling logistically and scientifically. In the case of GeneArt services, this includes preserving relative antibody expression levels for a reliable estimation of expression performance in re-orders and scale-ups.
5. High-quality, reproducible data
Reliable outsourcing services can help deliver precise analytics for volume, concentration, and purity, helping to ensure consistent expression and purification both within and across production runs. This consistency is critical for mitigating late-stage failures and controlling long-term development costs.
Another advantage of high-quality outsourcing services is the generation of robust datasets suitable for AI and machine learning applications. As computational approaches to protein design advance, the availability of accurate, high-quality data will be essential for optimizing antibody engineering and accelerating innovation.
For example, the GeneArt system underwent rigorous validation through a triplicate assessment of 38 pharmaceutical blockbuster antibodies. It also incorporates a Six Sigma quality management approach, which focuses on minimizing process variation and enhancing overall reliability.
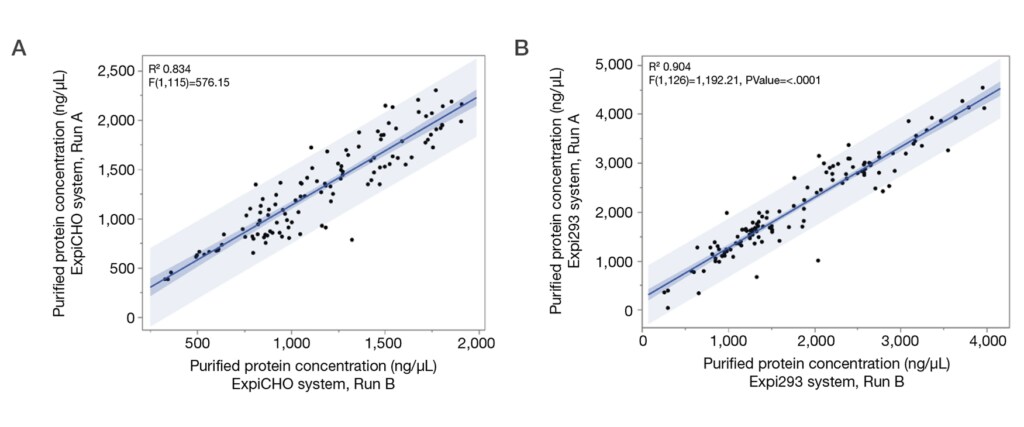
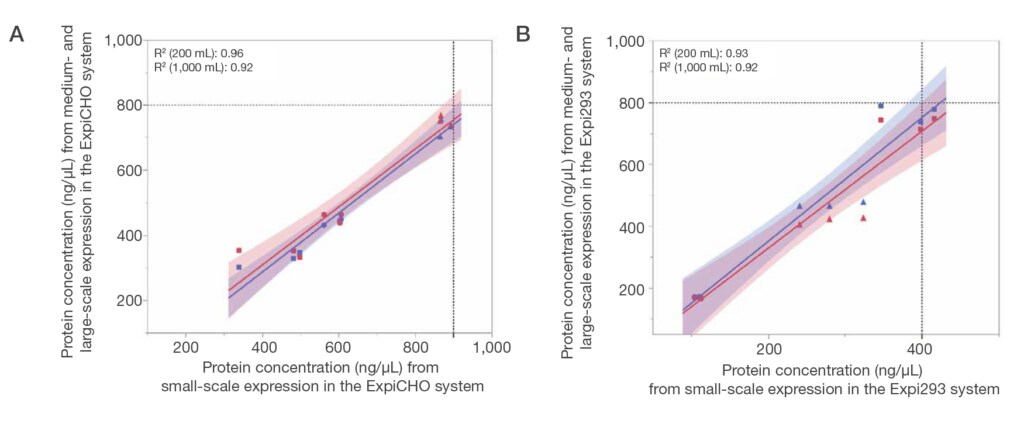
Potential drawbacks: should you always outsource antibody service?
Despite the clear benefits, outsourcing can come with risks:
- Loss of Control – Relying on an external partner can sometimes mean reduced oversight over proprietary data, timelines, and quality control. To help mitigate this challenge, GeneArt HTP Antibody Expression services aim for transparency through fully traceable Matrix™ 2D barcoded sample tubes, exceptionally high quality standards, and an on-call team of support staff for consistent project communication.
- Data Security Concerns – Intellectual property (IP) protection is crucial, requiring strict confidentiality agreements (CDAs/NDAs) with outsourcing partners. As a founding member of the International Gene Synthesis Consortium (IGSC) and an ISO9001 certified manufacturing site, Thermo Fisher Scientific is committed to strict biosecurity, biosafety, and confidentiality standards to give customers peace of mind.
- Long-Term Costs – While outsourcing saves money initially, long-term dependence on external providers may become expensive compared to building internal capabilities. However, building in-house capacity can be costly and may limit flexibility when needs fluctuate for different projects. Outsourcing provides a range of scales and throughputs that can be utilized only when needed.
Recombinant antibody production services can help speed discovery.
The demand for novel antibody-based therapeutics—and the upstream discovery workflows that support them—is growing fast. Researchers can engineer different aspects of an antibody’s structure to fine-tune its function and pharmacokinetics. While several methods exist, many are error-prone and require screening large numbers of candidates to identify viable drug products. This makes the process resource-intensive and significantly impacts downstream drug development.
With so much riding on the discovery phase and a high risk-to-reward ratio, more biopharmaceutical companies are turning to specialized service provider partners for protein drug discovery. The right service provider can provide access to advanced technologies helping teams generate more candidates, tap into broader technical expertise, and shorten development timelines. Ultimately, outsourcing these critical steps can help lead to a smarter use of resources, cutting costs and time while helping increase the chances of clinical success.
##
© 2025 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
For Research Use Only. Not for use in diagnostic procedures.
References
1. Waldmeier, L. et al. Transpo-mAb display: Transposition-mediated B cell display and functional screening of full-length IgG antibody libraries. MAbs 8, 726–740 (2016).
2. Delgado, M. & Garcia-Sanz, J. A. Therapeutic Monoclonal Antibodies against Cancer: Present and Future. Cells vol. 12 Preprint at https://doi.org/10.3390/cells12242837 (2023).
3. Puthenpurail, A., Rathi, H., Nauli, S. M. & Ally, A. A BRIEF SYNOPSIS OF MONOCLONAL ANTIBODY FOR THE TREATMENT OF VARIOUS GROUPS OF DISEASES HHS Public Access.
4. Desoubeaux, G. et al. Therapeutic monoclonal antibodies for respiratory diseases: Current challenges and perspectives, March 31 – April 1, 2016, Tours, France. MAbs 8, 999–1009 (2016).
5. Ke, Q., Kroger, C. J., Clark, M. & Tisch, R. M. Evolving Antibody Therapies for the Treatment of Type 1 Diabetes. Frontiers in Immunology vol. 11 Preprint at https://doi.org/10.3389/fimmu.2020.624568 (2021).
6. Zahavi, D. & Weiner, L. Monoclonal antibodies in cancer therapy. Antibodies vol. 9 1–20 Preprint at https://doi.org/10.3390/antib9030034 (2020).
7. Engineering Therapeutic Monoclonal Antibodies.
8. Lazar, G. A. et al. Engineered Antibody Fc Variants with Enhanced Effector Function. www.pnas.orgcgidoi10.1073pnas.0508123103 (2006).
9. Ramdani, Y., Lamamy, J., Watier, H. & Gouilleux-Gruart, V. Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review. International Journal of Molecular Sciences vol. 23 Preprint at https://doi.org/10.3390/ijms23179604 (2022).
10. Global Antibody Discovery Services Market Set to Soar Past $1.6 Billion by 2023: Aiding Pharmaceutical Efficiency Through Outsourcing. GlobeNewswire https://www.globenewswire.com/news-release/2023/10/18/2762141/28124/en/Global-Antibody-Discovery-Services-Market-Set-to-Soar-Past-1-6-Billion-by-2023-Aiding-Pharmaceutical-Efficiency-Through-Outsourcing.html (2023).



Leave a Reply