Multiplex immunoassays, including multiplex ELISA, are powerful tools for researchers seeking to analyze multiple biomarkers simultaneously.
This guide explores the principles, applications, and advantages of multiplex ELISA and other multiplex immunoassay techniques.
Table of contents
What are multiplex immunoassays?
Multiplex immunoassays are advanced techniques used in biomedical research to simultaneously measure multiple analytes (e.g., proteins, peptides, or antibodies) in a single well with a small sample volume (e.g. 1-50 µL) of plasma, serum, cell culture supernatants, or other bodily fluids. By allowing the detection of several biomarkers at once, these assays help provide a more comprehensive overview of biological processes and disease states compared to traditional single-analyte assays and are especially valuable when sample volume is limited.
Enzyme-Linked Immunosorbent Assay (ELISA) is a common and widely used method to perform sample analysis and can accurately detect and quantify individual proteins with high specificity and sensitivity. The selectivity of ELISA is achieved using qualified single- or double-antibody sandwich technology, and accurate quantitation using calibrated standards. ELISAs are performed in a 96-well format with results typically ready after ~4 hours on a microplate reader. Results obtained with ELISAs are generally reproducible and consistent with biologically relevant sensitivity levels and dynamic ranges.
While ELISA is a widely trusted method of protein analysis in general, multiplexing methods enable the measurement of multiple analytes simultaneously in a single well requiring smaller sample input and less hands-on time than ELISA, helping reduce time to results, cost and labor.
Common research applications of multiplex immunoassays
Multiplex immunoassays are widely utilized in various research fields, including:
- Biomarker Discovery: Identifying new biomarkers for diseases.
- Immune Response Studies: Understanding how the immune system responds to infections or treatments.
- Drug and Vaccine development: Measuring inflammatory and cytokine responses pre- and post-treatment
- Clinical Diagnostics: Diagnosing and monitoring diseases.
- Pharmacodynamics and Toxicology Studies: Assessing the effects of drugs and their safety profiles.
What are the different types of immunoassays?
There are several types of multiplex immunoassays available, and three are described below.
Electrochemiluminescence Immunoassay (ECLIA)
Electrochemiluminescence (ECL) assays use electrochemical and chemiluminescent principles to detect multiple analytes. This method offers high sensitivity and a wide dynamic range, making it suitable for applications requiring precise quantification of low-abundance biomarkers.
Olink PEA
The Olink Proximity Extension Assay (PEA) is a highly specific and sensitive technology that uses DNA-labeled antibody pairs to detect proteins. When the antibodies bind to their target, their DNA tags come into proximity and are extended, allowing for subsequent quantification using qPCR or next-generation sequencing (NGS). Olink PEA allows high multiplexing of up to 5,000+ proteins with high-specificity and high-sensitivity.
Luminex / Bead-Based
Luminex® xMAP® (multi-analyte profiling) technology uses color-coded beads, which are dyed with different concentration of fluorophores to generate bead sets that can be easily differentiated (Figure 1A). Individual bead sets are coated with specific antibodies to capture either one target analyte or two different targets on the same bead as used in Dual Reporter assays.
The captured analyte from a sample is detected using an analyte-specific biotinylated antibody that binds to the appropriate epitope of the immobilized analyte, plus streptavidin-conjugated R-phycoerythrin (S-RPE) or streptavidin-BV421 for Dual Reporter assay.
For detection of the immunoassay sandwich complex, Luminex™ instruments use either light-emitting diodes (LEDs) for excitation of each fluorescent bead combined with a CCD camera for bead and analyte detection (MagPix, discontinued), or a flow-based detection system using with a red and green laser for excitation of each fluorescent bead and measurement of the reporter dye (Luminex 200, FLEXMAP 3D, INTELLIFLEX, INTELLIFLEX DR-SE) and additional violet laser for measuring the second reporter in Dual Reporter assays (only available in INTELLIFLEX DR-SE).
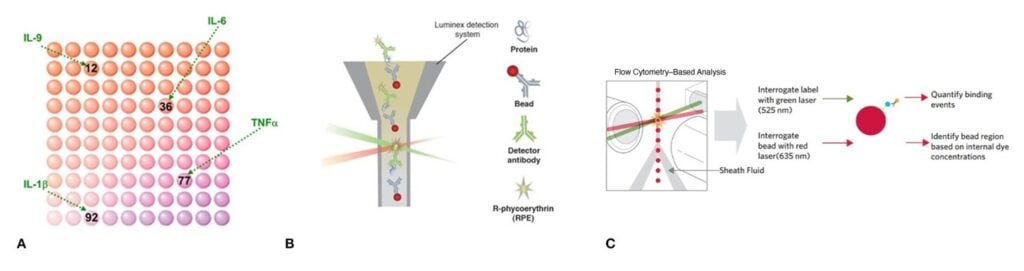
This Luminex technology is versatile and can be used for detecting both proteins and nucleic acids, making it a dual-capability platform for a wide range of applications in research. As each coated bead is individually identifiable for a specific analyte, multiple beads can be combined to simultaneously measure the levels of up to 500 targets for nucleic acid and typically no more than 80 targets for proteins due to biological interference in a single sample. Therefore, this bead-based approach allows for high-throughput and flexible multiplexing.
When to use a multiplex ELISA
While ELISA is a trusted method of protein analysis, multiplexing methods that enable the measurement of multiple analytes simultaneously in a single well address a number of specific limitations:
- ELISA allows for the measurement of only one analyte at a time in a given sample, limiting investigators’ increasing need to measure multiple targets for a holistic biological understanding of protein interactions.
- The limited sample volume available may limit the number of times analyses can be conducted. This is especially true in small animal research, in pediatric testing, and in microplate assays providing limited sample volume. The ability to assay multiple analytes in a single small-volume sample enables more effective use of each sample.
- Difficulties in data interpretation can arise when comparing analyte levels measured by multiple ELISAs, each assay having been performed with different sample aliquots and each subject to systematic errors leading to decreased precision and accuracy.
- Many analytes require assays with broad dynamic ranges to avoid repeat testing or out-of-range values. Multiplex assays can be designed to have large dynamic ranges for all of the analytes, or customer ranges tailored to various expected analyte concentrations.
The analysis of selected proteins in, for example, serum, plasma, cell lysates or urine is key to the study of inflammation, metabolism, cell signaling, cancer, cardiovascular disease, toxicology, neuroscience, and many other research and clinical areas. The ever-growing list of cytokines, chemokines, and other growth and differentiation factors, as well as their complex interactive networks in health and disease, has made it crucial to evaluate them in relevant groups rather than individually. Often, for example, the levels of cytokines or chemokines within a pathway in relation to each other are far more relevant than their presence or absence, or than the absolute levels of individual proteins.
Luminex™ xMAP™ assays have increasingly been adopted for sample analysis, and over 98,000 scientific publications are available demonstrating high precision and reproducible results. These studies have covered a broad range of common applications, including:
- Measurement of soluble analytes in serum, plasma, urine, cerebral spinal fluid (CSF), lavage fluids, cell culture supernatants and other sample types from human, monkey, mouse, rat, swine, canine and other species.
- Basic research—in vitro and in vivo studies
- Preclinical studies—in vitro and in vivo models
- Screening batches of sera for the presence/absence of defined markers or mediators
- Quantitative confirmation of data for targets identified from proteomic or genomic analysis
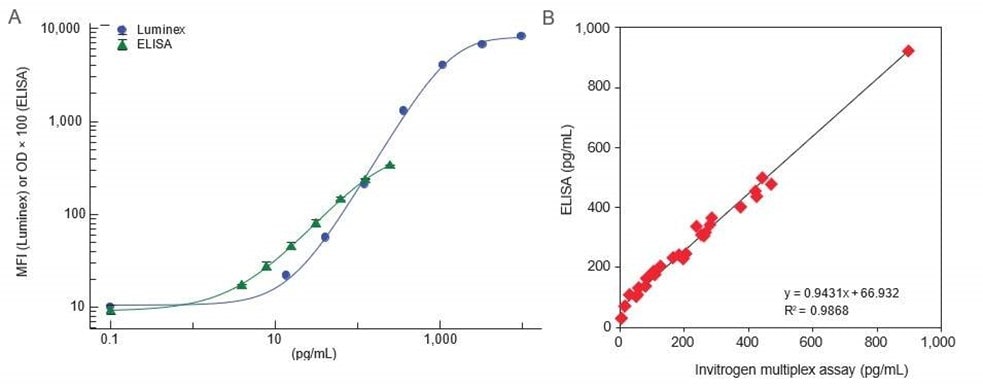
Invitrogen™ ProcartaPlex™ multiplex immunoassays are based on this Luminex xMAP technology, and provide a versatile platform that gives users more flexibility and a greater array of options for analyte detection.
Whether you are testing for single or multiple analytes, ProcartaPlex multiplex assays deliver accurate analytical performance using efficient, easy-to-follow protocols. These assays are developed by ELISA experts and undergo rigorous development, validation, manufacturing, and quality control processes comparable to our Invitrogen ELISAs.
In addition, multiplex assays undergo additional testing to ensure that there is no crosstalk between the assays. Each ProcartaPlex multiplex assay and ELISA assay is fully qualified with the sample type stated (i.e., species-specific serum, plasma, and cell culture supernatants), and each lot is evaluated based on the following performance characteristics:
- Specificity—each antibody pair is thoroughly tested in development to help ensure it detects the correct analyte and does not cross-react with other analytes
- Sensitivity—each analyte is evaluated for both functional sensitivity (differentiation from background) and lower limit of detection (LLOD)
- Precision/accuracy—multiplex assays have good intra-assay precision (<15% CV), inter-assay precision (<15% CV), and lot-to-lot consistency (<30% CV)
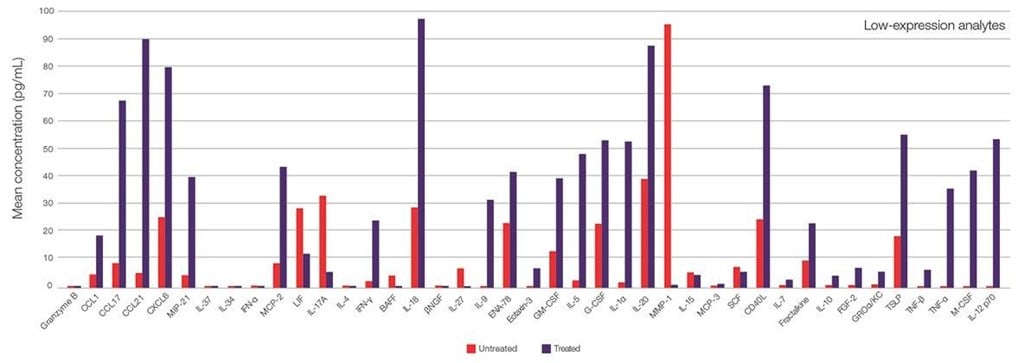
To demonstrate the power of multiplexing, 30 samples were analyzed: 23 untreated human serum samples and 7 plasma samples treated with 100ng/ml LPS for 20 hours . Expression pattern of low expressed targets are shown in the figure below. Data were generated using the Invitrogen™ ProcartaPlex™ Human Immune Response Panel, 80plex.
Multiplex immunoassay vs. ELISA: similarities and differences
Quality of results and reproducibility
Both multiplex immunoassays and traditional ELISA offer high-quality results. However, multiplex immunoassays can help provide more comprehensive data as they measure multiple analytes simultaneously, leading to better insights into complex biological systems. Reproducibility can be maintained across different runs, provided that the assays are well-validated.
Difficulty and time to perform
Multiplex immunoassays can be more complex to set up and optimize compared to single-analyte ELISA. However, once established, they save significant time by allowing the simultaneous measurement of multiple targets. This reduces the number of assays needed and speeds up data generation.
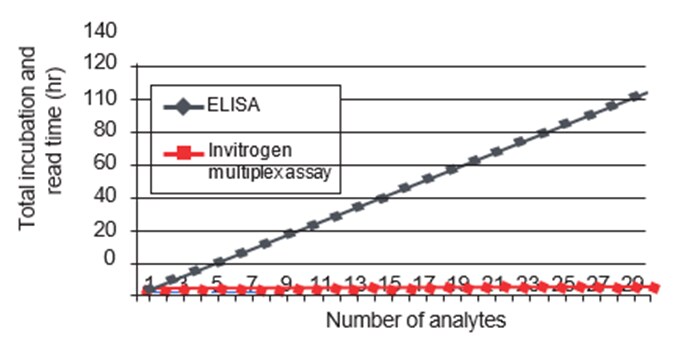
Cost
While the initial setup and reagents for multiplex immunoassays can be more expensive than single-analyte ELISA, the overall cost per analyte is often lower due to the reduced number of assays and samples required. The cost-effectiveness increases with the number of analytes measured.

Summary of the benefits of multiplexing immunoassays
The Luminex multiplex bead-based ProcartaPlex immunoassays have a number of significant advantages compared with traditional ELISA technology, including:
- Time savings—ProcartaPlex assays allow testing of up to 80 analytes in a single sample. In the same time it takes to set up one ELISA, multiple analytes can be measured, significantly reducing labor time.
- Smaller sample volume—depending on the expression levels, multiplex assays require 25-50 µL of sample or less for 384-well format, while still obtaining accurate results for all analytes.
- Broad dynamic range—provides the ability to reliably detect proteins across a broad concentration range.
- High throughput—the Luminex system reads from conventional 96-well and 384-well microtiter plate. Combined with the ability to read up to 80analytes per sample, this provides a high-throughput path to data collection.
Field Application Specialists (FAS) are available to educate you on multiplexing, answer questions, and help support all aspects of running and analyzing an Invitrogen multiplex assay. For any questions, please contact us at LuminexFAS@thermofisher.com or access the Multiplex Academy for a self-learning platform.
Learn more about the product in this blog:
Resources and Solutions for Luminex and ProcartaPlex immunoassay
- Luminex platform – Quantitative protein and RNA assays with superior throughput
- Luminex instrument and assay guide – High-Throughput multiplexing of protein and gene expression analysis
- ProcartaPlex Panel Configurator – This online tool helps you design a custom multiplex panel for your specific application
- ProcartaPlex Assays Support – Explore the “Getting Started” and “Troubleshooting” sections for solutions to top inquiries and common problems.
##
For Research Use Only. Not for use in diagnostic procedures.
© 2025 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.



Leave a Reply