PET, the World’s Most Recycled Plastic
 Polyethylene terephthalate (PET) is a strong, lightweight, transparent plastic. When PET is used to make fabric, it’s known as polyester, a popular material for sportswear because it is crease-resistant, tear-proof, weather-proof and hydrophobic. PET, or PET resin, is commonly used to make drink bottles as well as cosmetic and food-grade packaging including take-out and microwave safe food containers. Because of its good texture compatibility, PET is also used as basic material for blood vessel implants.
Polyethylene terephthalate (PET) is a strong, lightweight, transparent plastic. When PET is used to make fabric, it’s known as polyester, a popular material for sportswear because it is crease-resistant, tear-proof, weather-proof and hydrophobic. PET, or PET resin, is commonly used to make drink bottles as well as cosmetic and food-grade packaging including take-out and microwave safe food containers. Because of its good texture compatibility, PET is also used as basic material for blood vessel implants.
According to the PET Resin Association website, PET was first synthesized in North America in the mid-1940s by DuPont chemists, who branded their creation as “Dacron.” In the late 1950s, researchers created PET film, which is now used extensively for video, photo and packaging films. By the 1970s, PET was being molded into bottles. As the site points out, one of PET’s major advantages is that it’s 100% recyclable:
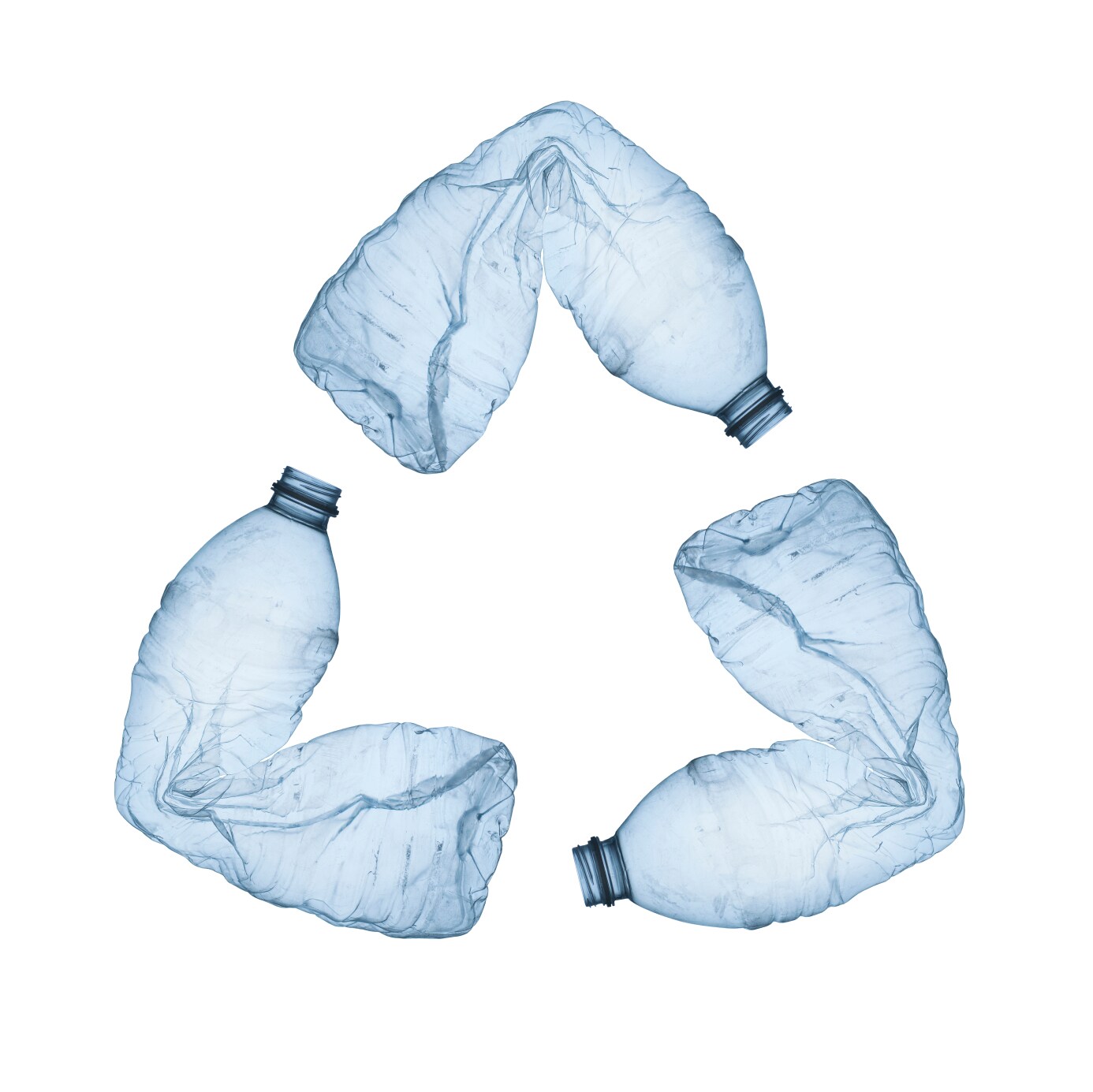
- PET is the most recycled plastic in the world, at a rate of 31% in the United States and 52% in Europe.
- PET is the only plastic that can be identified by the #1 code within a triangular recycling arrows symbol.
- PET can be commercially recycled by washing and re-melting, or by chemically breaking it down to its component materials to make new PET resin.
- Common recycled PET products include new PET bottles and jars, carpet, clothing, industrial strapping, rope, automotive parts, fiberfill for winter jackets and sleeping bags, construction materials, and protective packaging.
In addition to recyclability, another reason why PET is a good choice for food and drink packaging is that it doesn’t contain BPA or phthalates. The Committee of PET Manufacturers in Europe (CPME) website explains:
Phthalates are low molecular weight monoesters made from orthophthalic acid. By comparison, PET is a high molecular weight polyester made from terephthalic acid and these forms are chemically very different. Phthalates (i.e. phthalate ester plasticizers) are not used in the manufacture of PET, and PET itself is not a phthalate. Plasticizer phthalates are sometimes used to soften other types of plastic, but they are not used in PET. Some consumers may have incorrectly assumed that PET is a phthalate because PET’s chemical name is polyethylene terephthalate.
PET is made from terephthalic acid and ethylene glycol which are combined to form a polymer chain. PET is processed by melt extruding and then stretching the material up to six times the original length to form an endless high-yield point thread. To produce a product of constant quality, the processor needs to know how the flow characteristics of the molten polymer change as a function of the temperature and the shearing rate.
Read Investigation of the Flow Characteristics of PET at Different Temperatures to learn how we investigated these flow characteristics with a modular torque rheometer system, under conditions similar to those encountered during processing.




What plasticizer should be used for rPET?