The collection of reflected light by our eyes leads to the perception of an object’s color, specifically light in the visible range of the electromagnetic spectrum (~400 nm – 700 nm). As our eyes are sensitive to variations in color and brightness,1 small changes in the color of an object can be easily observed.
In pharmaceutical manufacturing, the color of a drug product is important to analyze for QA/QC purposes. Not only is it necessary to minimize batch-to-batch variations for aesthetic purposes, but changes to the color of a product can have implications for the quality of the products. Specifically, variations from the anticipated color could indicate impurities are present in the product or that the material has degraded.2–4 This is particularly important for materials which are easily decomposed, including light, moisture, and oxygen/air-sensitive substances.5 The figure below is a diagram of how the color of an object is perceived.

Qualitatively, a comparison of the color of a finished drug product with an accepted standard can be used to ensure the material’s color matches. However, inherently this methodology will introduce person-to-person variations.6 Additionally, environmental effects, such as the light source or the presence of shadows, can influence the perceived color.
There are ways to remedy that issue. As the color of a material comes from the reflected visible light, spectroscopic measurements of a material in the visible spectral range can be used to provide a more rigorous and quantitative method for assessing color.
A UV-Visible spectrophotometer can be used to measure either the percent of light transmitted (%T) or reflected (%R) across the visible spectrum for this purpose. UV-Vis spectrophotometers measure the transmission or reflection of light in the ultraviolet and/or visible region of the spectrum. As both %T and %R measurement geometries are possible, this instrument, and subsequent color analysis, can be applied to both liquid and solid products.
The American Society for Testing and Materials (ASTM),7 as well as USP <1061>,8 have detailed descriptions of the mathematics that can be used to assign the sample’s color a coordinate in a graphical representation of color, also referred to as a color space.
The tristimulus values, calculated through the equations 1 – 3 below,
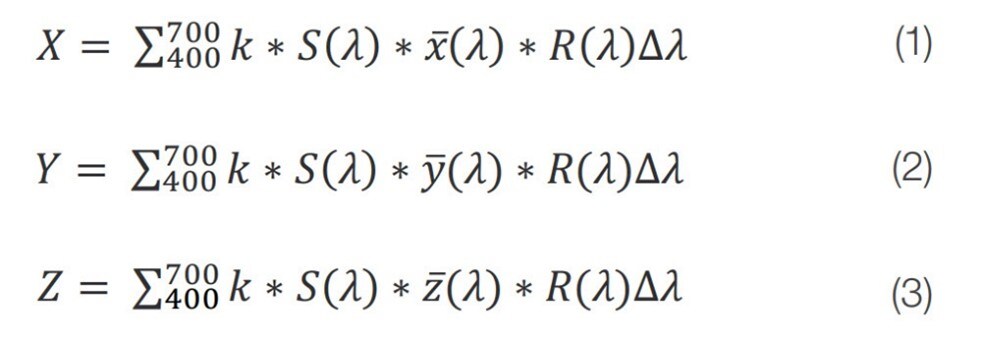
are the basis of most other color spaces developed by the Comission Internationale de l’Eclairage (CIE).9 These formulas include the measured reflectance (R(λ)), the spectral power of an illuminant (S(λ)), a color matching function (x(λ),y(λ),z(λ)), and the normalization factor (k). S(λ) and the color matching functions account for the light source under which the color is to be determined, as well as the field of view for the observer, respectively.
In pharmaceutical applications, specifically in QA/QC functions, the ability to compare the sample to an accepted standard, as well as establish acceptance criteria, is critical. Consequently, a uniform color space, CIE L*a*b*, must be used instead.
These issues and the accompanying experiments are discussed in an application note about color analysis and its implementation for both solid and liquid samples using UV-Vis spectrophotometers and the appropriate software. Read about the materials and instruments used, the parameters, and the results in Color analysis for pharmaceutical products using UV-Visible absorption techniques.
The experiments conducted will show that color analysis can be an effective and quick method for QA/QC in pharmaceutical manufacturing. The application note illustrates how color analysis can be performed using UV-Visible Spectrophotometers to carefully determine a material’s color without person-to-person variations, allowing for a quantitative analysis of a produced pharmaceutical. Additionally, these measurements demonstrate the ability to analyze both liquid and solid samples following USP color analysis procedures.
Resources, Notes, and References:
- Application note: Color analysis for pharmaceutical products using UV-Visible absorption techniques.
- For Research Use Only. Not for use in diagnostic procedures.
References
- Ng, S.E., Tay, Y.B.; Ho, T.Y.K.; Ankit; Mathews, N., Inorganic Electrochromic Transistors as Environmentally Adaptable Photodetectors, Nano Energy, 2022, 97, 107142.
- Zhou, L.; Vogt, F. G.; Overstreet, P. -A.; Dougherty, J. T.; Clawson, J. S.; Kord, A. S., A Systematic Method Development Strategy for Quantitative Color Measurement in Drug Substances, Starting Materials, and Synthetic Intermediates, J. Pharm. Innov., 2011, 6, 217 – 231.
- Yamazaki, N.; Taya, K.; Shimokawa, K.-I., Ishii, F., The Most Appropriate Storage Method in Unit-Dose Package and Correlation between Color Change and Decomposition Rate of Aspirin Tablets, Int. J. Pharm., 2010, 396, 105 – 110.
- Oram, P. D.; Strine, J., Color Measurement of a Solid Active Pharmaceutical Ingredient as an Aid to Identifying Key Process Parameters, J. Pharm. Biomed. Anal., 2006, 40, 1021 – 1024.
- Berberich, J., Dee, K.-H., Hayauchi, Y., Pörtner, C., A New Method to Determine Discoloration Kinetics of Uncoated White Tablets Occurring During Stability Testing – An Application of Instrumental Color Measurements in the Development Pharmaceutics, Int. J. Pharm., 2002, 234, 55 – 66.
- Hetrick, E. M.; Vannoy, J.; Montgomery, L. L.; Pack, B. W., Integrating Tristimulus Colorimetry into Pharmaceutical Development for Color Selection and Physical Appearance Control: A Quality-by-Design Approach, J. Pharm. Sci., 2013, 102, 2608 – 2621.
- ASTM International. Standard Practice for Computing the Color of Objects by Using the CIE System; ASTM E308-08; West Conshohocken, PA.
- United States Pharmacopeia and National Formulary. <1061> Color – Instrumental Measurement. In: USP–NF. Rockville, MD: USP
- Subert, J.; Cizmarik, J., Application of Instrumental Colour Measurements in Development and Quality Control of Drugs and Pharmaceutical Excipients, Pharmazie, 2008, 63, 331 – 336.
Leave a Reply