|
You can now learn on the go. Click play to listen to this blog:
|
In Vitro Diagnostic Medical Device Directive is Being Replaced
Here’s What that Means for Diagnostic Device Classification
The European Union’s new In Vitro Diagnostic Medical Device Regulation (IVDR) becomes law near the end of May this year, replacing the previous regulatory regime, the In Vitro Diagnostic Medical Device Directive (IVDD). Diagnostic devices sold in the European Union (EU) will face a new regulatory classification system with new categories, which change the standards manufacturers must meet. So, what does this mean in practice?
IVDD Regulatory Classification System Explained
Predetermined List of Medical Conditions
The IVDD’s regulatory classification was based on which conditions or pathogens a given diagnostic medical device was designed to diagnose. It grouped those conditions into several extensive lists and annexes. Different lists and annexes within the IVDD corresponded to different levels of regulatory scrutiny and different standards that products would have to meet.
Because the IVDD relied on a predetermined list of medical conditions, it was unable to respond effectively to both the emergence of new deadly pathogens and the self-administered diagnostic tests associated with them deployed on a wide scale. The current situation demanded a different regulatory regime, which the IVDR aims to provide.
IVDR – Risk, Not List
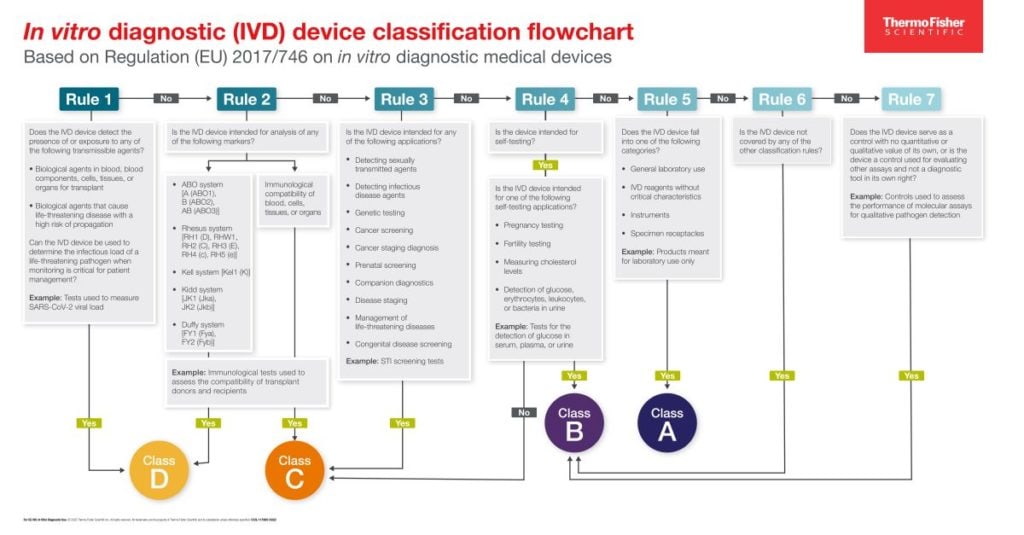
In an attempt to avoid these problems going forward, the IVDR instead uses a risk-based classification scheme. This scheme assigns diagnostic medical devices to one of four classes (A through D) based on ascending risk to patients if the device malfunctions, using seven rules to assess such risk. Higher-risk products will have more stringent standards for approval than lower-risk products. Briskly summarized:
- Rule 1 asks whether the device is used for detection of high-risk disease in blood or tissues, such as a test used to screen blood or tissue donations for syphilis.
- Rule 2 asks whether the device is used for testing blood or tissue compatibility and whether a high-risk blood group is in play, such as immunological tests used during organ transplantation.
- Rule 3 asks whether the device is used for infectious diseases, cancer, genetics, congenital screening, or companion diagnostics, such as STI screening.
- Rule 4 asks whether the test is a self-test or near-patient test for a high-risk disease and whether the test is for fertility, pregnancy, or cholesterol, such as an at-home pregnancy test.
- Rule 5 asks whether the test is a specific IVD-related reagent, instrument, or specimen receptacle. These are primarily products meant for laboratory use.
- Rule 6 asks whether the device is not covered by the previous rules.
- Rule 7 asks whether the device is a control used for interpreting other assays rather than a diagnostic tool in its own right.
This summary is not the complete text of the IVDR rules and should not be taken as such.
Did My Product Classification Change?
Products Regulated Under IVDD
Most products regulated under the IVDD received the lowest-risk classification that directive offered, “IVD Others,” which does not require the approval or involvement of a notified body. Van Drongelen et al1 estimate [PDF] that 93.1% of all products regulated under the IVDD received this classification, and only 0.9% of products received the strictest classification, “List A.”
Products Regulated Under IVDR
Under the IVDR, this will change markedly. Van Drongelen et al estimate that only 15.9% of medical diagnostic products will receive this low-risk rating equivalent under the IVDR (class A), and 84.2% will instead receive one of the higher-risk classifications. These higher-risk ratings require notifying an approving body to assess the manufacturing and quality-control process in order to assure safety of the product and compliance with the IVDR’s requirements for that class. Thus, many products that did not require extensive regulatory approval processes under the IVDD will have to be re-approved under the IVDR.
Example: SARS-CoV-2 Diagnostic Tests
An example that illustrates the change in regulatory priorities is SARS-CoV-2 diagnostic tests. Since SARS-CoV-2 is not listed in the IVDD, these tests received the lowest level of regulatory scrutiny, requiring an EC Declaration of Conformity that did not need to be verified by any regulatory agency. Under the IVDR, however, they are classified as class D products due to being tests for a high-risk pathogen for the patient and for the wider population and therefore face the highest standards for approval the IVDR requires.
Higher Standards Designed to Improve Public Health and Safety
These higher standards are designed to improve public health and safety as the number and importance of diagnostic medical devices continues to increase, but they will also create hurdles for assay manufacturers, as a considerable amount of time and effort will need to be expended in order to bring their IVDD products into compliance under the IVDR while minimizing market disruption.
For products previously regulated under the IVDD that already required some manner of verification by a notifying body, such as tests for phenylketonuria (Annex II List B) and hepatitis B (Annex II List A), compliance with the IVDR will be much more similar to the existing qualification process of the IVDD.
Personalized Transition Assistance & Plans
Thermo Fisher Scientific has IVD transition assistance and promotions that you may qualify for.
Contact Us Today
Contact the Thermo Fisher Scientific transition team about a personalized IVD transition plan.
- Please specify in the subject line the specific nature of your inquiry.
Contact our OEM and Commercial Licensing team
- Learn more about how you can benefit from an OEM partnership.
Related Links & Resources
- Three Steps You Can Take to Navigate the IVDR
- Learn more about the In Vitro Diagnostic Regulation (IVDR)
- Getting Ready for the IVDR
- Genetic Sciences OEM and Commercial Supply – Your innovation deserves every advantage
—
Citations
- Van Drongelen A, A De Bruijn, J Pennings, et al. (2018) The impact of the new European IVD-classification rules on the notified body involvement; Natl. Inst. Public Health Environ. Minist. Health Welf. Sport.

Leave a Reply