Search
LIVE/DEAD Yeast Viability Kit Protocol

Two-color assay distinguishes live and dead yeast
This kit combines a novel two-color fluorescent probe for yeast viability, FUN 1, with a fluorescent fungal surface labeling reagent, Calcofluor White M2R. Plasma membrane integrity and metabolic function of fungi are required to convert the yellow-green fluorescent intracellular staining of FUN 1 into red-orange intravacuolar structures; Calcofluor white M2R labels cell-wall chitin with blue-fluorescence, regardless of metabolic state.
This protocol can be used for:
- Identifying live and dead yeast using a fluorescence microscope
This protocol should not be used for:
- Flow cytometry
You will need the following for this protocol:
- Yeast growing in culture
- LIVE/DEAD Yeast Viability Kit, for microscopy (Cat. No. L7009)
- Medium such as yeast extract peptone dextrose (YPD) (Cat. No. A1374501)
- Wash buffer such as 10 mM Na-HEPES (pH 7.2) with 2% D-glucose
- Fluorescence microscope
Protocol
1. Preparation of yeast suspensions
2. Staining yeast
Spectral information and storage
| FUN 1 cell stain | Calcofluor white M2R | |
|---|---|---|
| Excitation/Emission | 488/530 nm | 365/435 nm |
| Standard filter set | FITC | DAPI |
| Storage conditions | ≤20°C, protect from light | ≤20°C, protect from light |
Protocol tips
- Warm vials to room temperature and centrifuge briefly before opening
- Wash to remove all growth medium from yeast before staining
- Phosphate wash buffers may decrease staining efficiency and are not recommended
- If precipitate is observed in Calcofluor dye, briefly centrifuge at 10,000 x g to clear solution
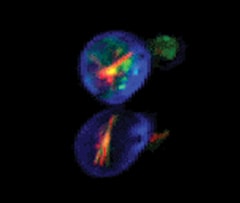
Saccharomyces cerevisiae stained with FUN 1 cell stain, which generates red-fluorescent intravacuolar structures, and with Calcofluor white M2R, a blue-fluorescent cell wall stain.