Mineral oil hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH) have moved from niche laboratory concerns to central topics in food safety, regulatory policy, and analytical science.
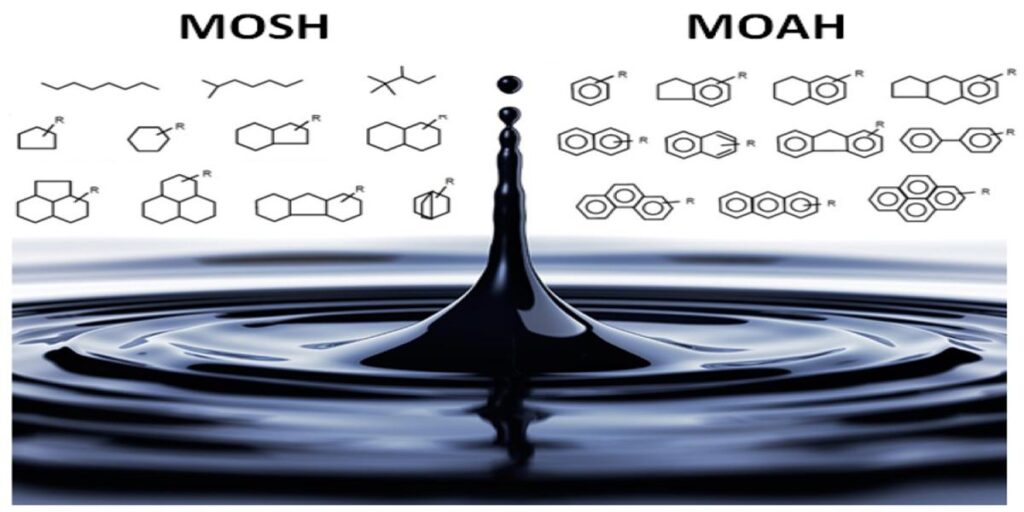
These compounds—originating from sources such as environmental pollution, lubricants, processing aids, and migration from packaging materials—can enter the food chain and accumulate in both raw ingredients and finished products.
- MOSH are saturated hydrocarbons, which can accumulate in human tissues but are generally considered of lower toxicological concern.
- MOAH contain aromatic rings, including polycyclic structures; certain high-ring MOAH are suspected to have genotoxic and carcinogenic properties, making them a priority for regulatory control.
Growing public awareness, coupled with scientific research and legislative pressure, is pushing the food industry toward more robust monitoring and testing.
The evolving regulatory landscape
For years, MOSH/MOAH testing lacked consistent rules across jurisdictions. Different countries and organizations used their own limits and methods, creating confusion for global supply chains. Over the last decade, several important steps have brought the field closer to harmonization:
- 2017 – EN 16995 (CEN)
The first standardized method for quantifying MOSH and MOAH in foodstuffs using LC-GC-FID technology. - 2023 – JRC Guidelines v2
Expanded scope to include food contact materials, with refinements in sample preparation and measurement. - 2024 – ISO 20122:2024
A dedicated standard for fats and oils, integrating clean-up procedures alongside LC-GC-FID to improve accuracy. - Ongoing – EURL/ISO initiatives
Collaborative efforts including interlaboratory studies, method refinement, and the potential adoption of GC×GC–MS for enhanced selectivity and molecular insight.
A major development still in progress is the EU’s draft regulation for maximum residue limits for MOAH. Expected after 2024, it could introduce strict, enforceable thresholds across the single market, forcing a step-change in testing capacity and method validation.
Analytical challenges in MOSH/MOAH testing
Although standardized methods exist, MOSH/MOAH analysis is not a straightforward “plug-and-play” process. Laboratories face several persistent challenges:
- Complex sample matrices
Edible oils, processed foods, and packaging extracts contain a mix of natural compounds such as carotenoids, terpenes, and squalene. These can overlap chromatographically with MOSH/MOAH peaks, making quantification more difficult. - False positives and overestimation
Non-MOSH/MOAH compounds can mimic the target analytes, especially in less selective detection modes, leading to inflated results. - Low detection limits
Many regulations demand quantification in the low mg/kg range. Achieving this requires both clean sample extracts and highly sensitive analytical instrumentation. - Reproducibility across labs
Differences in sample handling, clean-up techniques, and instrument tuning can lead to significant interlaboratory variation, complicating compliance and enforcement.
These challenges mean that achieving accuracy, repeatability, and regulatory compliance requires optimized workflows that combine precision sample preparation with advanced chromatographic techniques.
A modern workflow: from sample to result
State-of-the-art MOSH/MOAH workflows are typically built around an LC-GC-FID platform supported by automated sample preparation. This integration reduces human error, improves throughput, and delivers reproducible results.
Step 1: saponification
Fats can overwhelm LC columns, limiting injection volumes and obscuring MOSH/MOAH peaks. Saponification removes these fats, enabling:
- Larger injection volumes.
- Clearer chromatographic baselines.
- Lower quantitation limits without compromising peak integrity.
Step 2: epoxidation
For MOAH analysis, carotenoids, terpenes, and other naturally occurring compounds must be removed. Epoxidation—now most commonly performed using PFA reagents—cleans up these interferences while maintaining the integrity of aromatic hydrocarbons.
Step 3: evaporation and concentration
By concentrating the hexane extract after saponification, laboratories can:
- Achieve lower detection limits.
- Improve recovery from challenging matrices such as palm stearates.
Step 4: alu oxide clean-up
Adding an alumina oxide clean-up step can substantially improve MOSH quantification in the presence of n-alkanes. This step is matrix-dependent and should be avoided if n-alkanes are absent to prevent bias.
Step 5: fraction collection for advanced analysis
When further identification is required—such as distinguishing between mineral oil hydrocarbons and plasticizers (POSH)—fraction collection followed by GC×GC-MS analysis can provide a deeper chemical fingerprint.
Beyond “how much”: the role of GC×GC-MS
Conventional LC-GC-FID answers the fundamental question: How much MOSH or MOAH is present in the sample?
But it cannot always explain the source or the chemical nature of the detected hydrocarbons.
This is where GC×GC-MS adds value:
- Source tracing – Identifies whether contamination originates from packaging, processing equipment, or environmental exposure.
- Structural characterization – Distinguishes low-ring MOAH (less concerning) from high-ring MOAH (more concerning toxicologically).
- False positive elimination – Confirms the identity of suspect peaks, reducing the risk of misreporting.
By combining FID for precise quantification with MS for molecular identification, GC×GC-MS enables both regulatory compliance and investigative insight.
The role of chromeleon software in MOSH/MOAH analysis
Modern analytical systems rely heavily on software to ensure speed, traceability, and accuracy. Chromeleon software offers:
- Unified control of LC, GC, and sample preparation hardware from a single interface.
- Automated workflow setup – In just a few clicks, vial positions, injection types, and processing methods are assigned.
- Integrated quality checks – Internal standard verification and retention time monitoring are built-in.
- Customizable, regulatory-ready reports – Exportable in PDF, CSV, XML, and other formats without external spreadsheets.
The result is streamlined operation, reduced analyst intervention, and consistent compliance with good laboratory practice (GLP) requirements.
Looking ahead
With upcoming EU regulations likely to set strict MOAH limits, and with continued scientific scrutiny of MOSH accumulation, the demand for high-quality testing will only increase.
Key trends shaping the future of MOSH/MOAH analysis include:
- Wider adoption of GC×GC-MS for confirmation and source identification.
- Greater automation in sample preparation to increase throughput and reproducibility.
- Regulatory harmonization, which will help reduce discrepancies between international markets.
- Lower detection thresholds, requiring even more sensitive and selective methods.
For laboratories, the challenge is twofold: staying ahead of compliance requirements while building capacity for high-volume, high-accuracy testing. Those that invest in robust, automated, and harmonized workflows now will be better positioned to meet both current and future demands.
Resources:
- TriPlus RSH Autosampler and Liquid Handling System
- TRACE 1600 Series Gas Chromatograph
- ISQ 7610 Single Quadrupole GC-MS
- Vanquish HPLC and UHPLC Systems
- Chromatography Data System (CDS)
- SureSTART 2 mL GOLD-Grade Glass Screw and Crimp Top Vials, Level 3 High Performance Applications
Please register for the free webinar on the 4th of September 2025 at 11 am Berlin time
Acknowledgement: Jorn Hofsteenge, SampleQ, Breda, The Netherlands
Visit us on LinkedIn: #MOSHMOAH #GCXGC #GCMS #Food



